|
|
|
|
Year of first isolation: |
1969 |
| Formula: | C29H46O8 |
| Molecular weight: | 522 |
| Occurence in plants: |
Vitex megapotamica [Lamiaceae (alt. Labiatae)] »  ![Wikipedia: Vitex megapotamica [Lamiaceae (alt. Labiatae)]](/images/wikipedia.png)
Serratula sogdiana [Asteraceae] »  ![Wikipedia: Serratula sogdiana [Asteraceae]](/images/wikipedia.png)
Lychnis flos-cuculi [Caryophyllaceae] »  ![Wikipedia: Lychnis flos-cuculi [Caryophyllaceae]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
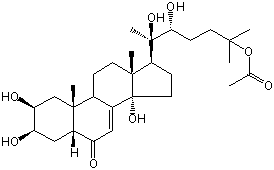
| |
| Canonical SMILES | CC(=O)OC(C)(C)CCC(C(C)(C1CCC2(C1(CCC3C2=CC(=O)C4C3(CC(C(C4)O)O)C)C)O)O)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@](C)([C@@H](CCC(C)(C)OC(=O)C)O)O)O)C)C » 
| | IUPAC Name | [(5R,6R)-5,6-dihydroxy-2-methyl-6-[(2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-6-oxo-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-17-yl]heptan-2-yl] acetate | | CAS-RN | 22033-96-1 | | PubChem CID | 11226211 | InChiKey
[ ChemIDPlus: search ] | DWHBRFSKXQCVDN-FORVDKSSSA-N | | InChI | InChI=1S/C29H46O8/c1-16(30)37-25(2,3)10-9-24(34)28(6,35)23-8-12-29(36)18-13-20(31)19-14-21(32)22(33)15-26(19,4)17(18)7-11-27(23,29)5/h13,17,19,21-24,32-36H,7-12,14-15H2,1-6H3/t17-,19-,21+,22-,23-,24+,26+,27+,28+,29+/m0/s1 |
| |
| CI-MS (NH3) m/z | 523 (M+H)+, 505, 480, 463 (MH-60)+, 445, 427, 409, 363, 345 | | EI-MS m/z (relative intensity %) | 504 (M-18)+, 486, 468, 462 (M-60)+, 444, 426, 411, 408, 393, 363, 345, 300, 99, 81 | | HR-MS | |
|
|
| C5D5N | | 01 | | | 02 | | | 03 | | | 04 | | | 05 | | | 06 | | | 07 | | | 08 | | | 09 | | | 10 | | | 11 | | | 12 | | | 13 | | | 14 | | | 15 | | | 16 | | | 17 | | | 18 | | | 19 | | | 20 | | | 21 | | | 22 | | | 23 | | | 24 | | | 25 | | | 26 | | | 27 | | | 28 | | | 29 | |
|
| C5D5N | | 01-Ha | 1.40 | | 01-He | 1.85 | | 02-Ha | 3.86 (m, w1/2=22) | | 03-He | 4.02 (m, w1/2=8) | | 04-Ha | 1.6 | | 04-He | 1.8 | | 05-H | 2.43 (dd ) | | 07-H | 5.84 (d, 2.5) | | 09-Ha | 2.99 (m, w1/2=22) | | 11-Ha | 1.70 | | 11-He | 1.78 | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | 2.0 | | 16-Hb | 1.72 | | 17-H | 2.33 (m ) | | 18-Me | 1.20 (s ) | | 19-Me | 1.07 (s ) | | 21-Me | 1.60 (s ) | | 22-Hb | 3.37 (d ) | | 23-Ha | 1.2 | | 23-Hb | | | 24-Ha | 2.17 | | 24-Hb | | | 26-Me | 1.45 (s ) | | 27-Me | 1.52 (s ) | | O-Ac | 1.95 (s ) |
| CDCl3 | | 01-Ha | | | 01-He | | | 02-Ha | | | 03-He | | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | | | 09-Ha | | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 0.85 (s ) | | 19-Me | 0.97 (s ) | | 21-Me | 1.20 (s ) | | 22-Hb | | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.40 (s ) | | 27-Me | 1.44 (s ) | | O-Ac | 1.95 (s ) |
|
| |
| M.P. | 198-199°C | | [α]D20 | + 60° (c 1.31; MeOH) | | IR (KBr) ν max (cm-1) | 1720 (C=O), 1650 (cyclohexenone), 1615 (C=C) | | UV (EtOH) λ max (log ε) | 243 (4.04) |
| |
| HPTLC | | | TLC | NP-TLC on silica gel : Rf 0.81 (CHCl3-EtOH 4:1); Rf 0.40 (EtOAc-MeOH-NH4OH 85:10:5); Rf 0.43 (CH2Cl2-EtOH 85:15) | | GLC | | | NP-HPLC | (Zorbax-SIL) Ret 10.6 min (CH2Cl2-iPrOH-H2O 125:30:2, 1 ml.min-1); | | RP-HPLC | (Spherisorb-5ODS2) Ret 18.4 min (ACN 0.1% TFA 23:77, 1 ml.min-1) |
| |
Drosophila melanogaster BII cell assay: EC50 = 1.0 x 10-7M | Drosophila melanogaster Kc-H cell assay: EC50 = 7.0 x 10-8M | Calliphora assay: 7% (20-hydroxyecdysone = 100%) |
| |
| First isolation | RIMPLER, H. et al. (1969) Tetrahedron Lett. (5), 329-333 |
 | | General | ZATSNY, I.L. et al. (1973) Khim. Prir. Soedin., 175-178 |
 | | General | ZATSNY, I.L. et al. (1975) Khim. Prir. Soedin., 155-158 |
 | | Bioactivities | CHERBAS, L. et al. (1980) W. Roux Arch. Devel. Biol. 189, 1-15 |
 | | Bioactivities | BERGAMASCO, R. et al. (1980) In: Progress in Ecdysone Research (Ed. J.A. Hoffmann), Elsevier/North Holland Biomed. Press, Amsterdam 299-324 |
 | | General | SUKSAMRARN, A. et al. (1995) Tetrahedron 51, 10633-10650 |
 | | Bioactivities | DINAN, L. et al. (1999) J. Computer-aided Mol. Design 13, 185-207 |
 |
| |
Permanent link to this datasheet: VITICOSTERONE E
|
|




![Wikipedia: Vitex megapotamica [Lamiaceae (alt. Labiatae)]](/images/wikipedia.png)

