|
|
|
|
Year of first isolation: |
1995 |
| Formula: | C28H46O8 |
| Molecular weight: | 510 |
| Occurence in plants: |
Paxillus atrotomentosus [Fungi] »  ![Wikipedia: Paxillus atrotomentosus [Fungi]](/images/wikipedia.png)
Ajuga turkestanica [Lamiaceae (alt. Labiatae)] »  ![Wikipedia: Ajuga turkestanica [Lamiaceae (alt. Labiatae)]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
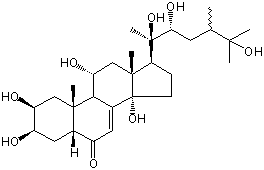
| |
| Canonical SMILES | CC(CC(C(C)(C1CCC2(C1(CC(C3C2=CC(=O)C4C3(CC(C(C4)O)O)C)O)C)O)O)O)C(C)(C)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2[C@@H](C[C@]2([C@]1(CC[C@@H]2[C@](C)([C@@H](CC(C(C)(C)O)C)O)O)O)C)O)C » 
C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2[C@@H](C[C@]2([C@]1(CC[C@@H]2[C@](C)([C@@H](C[C@H](C(C)(C)O)C)O)O)O)C)O)C » 
C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2[C@@H](C[C@]2([C@]1(CC[C@@H]2[C@](C)([C@@H](C[C@@H](C(C)(C)O)C)O)O)O)C)O)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,11R,13R,14S,17S)-2,3,11,14-tetrahydroxy-10,13-dimethyl-17-[(2R,3R,5S)-2,3,6-trihydroxy-5,6-dimethylheptan-2-yl]-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | | | PubChem CID | 15839570 | InChiKey
[ ChemIDPlus: search ] | FSNRQNKYLJDXMZ-APCZMLFGSA-N | | InChI | InChI=1S/C28H46O8/c1-14(24(2,3)34)9-22(33)27(6,35)21-7-8-28(36)16-11-17(29)15-10-18(30)19(31)12-25(15,4)23(16)20(32)13-26(21,28)5/h11,14-15,18-23,30-36H,7-10,12-13H2,1-6H3/t14-,15-,18+,19-,20+,21-,22+,23+,25-,26+,27+,28+/m0/s1 |
| |
| FAB-MS m/z | 533 (M+Na)+ (2), 511 (M+H)+ (4), 493 (12), 475 (13), 457 (11), 439 (3), 387 (2), 379 (2), 373 (2), 362 (4), 345 (5), 317 (6), 299 (9), 281 (5), 249 (7), 225 (8), 213 (10), 197 (12), 181 (23), 157 (25), 113 (75), 93 (100), 83 (71), 69 (70), 57 (95) | | HRESI-MS | 533.30800 [M+Na], expected 533.30849 for C28H46O8Na | | ESI-MS m/z | 511 [M+H], 493 [MH-H2O], 475 [MH-2H2O], 457 [MH-3H2O], 439 [MH-4H2O] |
|
|
| CD3OD | | 01 | 39.07 | | 02 | 68.94 | | 03 | 68.56 | | 04 | 33.28 | | 05 | 52.78 | | 06 | 206.72 | | 07 | 122.78 | | 08 | 165.70 | | 09 | 42.93 | | 10 | 39.90 | | 11 | 69.50 | | 12 | 43.75 | | 13 | a | | 14 | 84.95 | | 15 | 31.83 | | 16 | 21.61 | | 17 | 50.20 | | 18 | 18.85 | | 19 | 24.60 | | 20 | 77.96 | | 21 | 20.66 | | 22 | 77.88 | | 23 | 35.12 | | 24 | 44.42 | | 25 | 74.07 | | 26 | 28.19 | | 27 | 25.86 | | 28 | 16.89 |
| D2O | | 01 | 39.1 | | 02 | 69.3 | | 03 | 69.3 | | 04 | 33.7 | | 05 | 53.4 | | 06 | 210.4 | | 07 | 123.9 | | 08 | 167.6 | | 09 | 43.4 | | 10 | 40.7 | | 11 | 70.3 | | 12 | 43.9 | | 13 | 49.4 | | 14 | 86.6 | | 15 | 32.4 | | 16 | 22.0 | | 17 | 50.8 | | 18 | 19.7 | | 19 | 25.3 | | 20 | 80.2 | | 21 | 21.5 | | 22 | 76.1 | | 23 | 35.2 | | 24 | 42.0 | | 25 | 76.4 | | 26 | 27.3 | | 27 | 27.7 | | 28 | 15.1 |
|
| CD3OD | | 01-Ha | 1.38 (dd, 13.0, 12.0) | | 01-He | 2.59 (dd, 13.0, 4.2) | | 02-Ha | 4.01 (ddd, 12.0, 4.2, 3.5) | | 03-He | 3.96 (q ) | | 04-Ha | 1.78 (dt, 14.0, 13.2, 2.5) | | 04-He | 1.68 (dt, 14.0, 4.0, 2.5) | | 05-H | 2.34 (dd, 13.2, 4.0) | | 07-H | 5.80 (dd, 2.7, 0.8) | | 09-H | 3.14 (dd, 8.8, 2.7) | | 11-Ha | 4.10 (ddd, 10.5, 8.8, 6.0) | | 12-Ha | 2.21 (dd, 12.2, 10.5) | | 12-He | 2.15 (dd, 12.2, 6.0) | | 15-Ha | 1.95 | | 15-Hb | 1.57 | | 16-Ha | 2.00 | | 16-Hb | 1.79 | | 17-H | 2.42 (dd, 9.6, 8.6) | | 18-Me | 0.874 (s ) | | 19-Me | 1.056 (s ) | | 21-Me | 1.195 (s ) | | 22-H | 3.47 (dd, 9.4, 2.0) | | 23-Ha | 1.86 (ddd, 14.4, 4.2, 2.0) | | 23-Hb | 1.05 | | 24-H | 1.65 (dp, 4.2, 7.0, 7.0, 7.0, 7.0) | | 26-Me | 1.142 (s ) | | 27-Me | 1.211 (s ) | | 28-Me | 1.034 (d, 7.0) |
| D2O | | 01-Ha | 1.39 (t, 13) | | 01-He | 2.48 (dd, 13, 3.7) | | 02-Ha | 4.09 (m, w1/2 =11) | | 03-He | 4.09 (m, w1/2 =11) | | 04-Ha | 1.74 | | 04-He | 1.79 | | 05-H | 2.31 | | 07-H | 5.98 (d, 2.6) | | 09-Ha | 3.13 (dd, 8.8, 3.6) | | 11-Ha | 4.23 (m, w1/2 =27, ddd, 10.8, 9, 6.1) | | 12-Ha | 2.05 | | 12-He | 2.28 (dd, 12.7, 6) | | 15-Ha | 2.06 | | 15-Hb | 1.65 | | 16-Ha | 1.90* | | 16-Hb | 1.84* | | 17-H | 2.32 (m) | | 18-Me | 0.87 (s) | | 19-Me | 1.09 (s) | | 20-H | | | 21-Me | 1.26 (s) | | 22-H | 3.55 (d, 11) | | 23-Ha | 1.25 | | 23-Hb | 1.52 | | 24-Ha | 1.73 | | 26-Me | 1.18 (s) | | 27-Me | 1.20 (s) | | 28-Me | 0.96 (d, 6.8) |
|
| |
| M.P. | | | [α]D20 | | | IR (KBr) ν max (cm-1) | 3404 (OH), 1657 (cyclohexenone), 1054, 1072 (C-O) | | UV (EtOH) λ max (log ε) | |
| |
| NP-HPLC | column Silasorb 600 (4 x 250 mm), flow-rate 0.8 ml.min-1, solvent Et2O-CH3CN-H2O (780:190: 30), Ret 50.5 min (20E 31.7 min) | | HPTLC | | | TLC | | | GLC | | | HPLC | RP-HPLC : column Separon SIX C18 (4 x 250 mm), solvent MeOH-H2O, linear gradient 10 to 70 % MeOH in 50 min, flow-rate 0.6 ml.min-1, Ret 41.1 min (20E 42.7 min). |
| |
Drosophila melanogaster BII cell assay: EC50 = 1.0 x 10-5M |
| |
| First isolation | VOKÁČ, K. et al. (1995) 16th Conference on Isoprenoids, Prague, Abstracts, pp. 77-78 |
 | | Bioactivities | HARMATHA, J. et al. (1997) Arch. Insect Biochem. Physiol. 35, 219-225 |
 | | Identification | VOKÁČ, K. et al. (1998) Tetrahedron 54, 1657-1666 |
 | | General | GUIBOUT, L. et al. (2015) Phytochemical Analysis 26, 293-300 |
 |
| |
Permanent link to this datasheet: 25-HYDROXYATROTOSTERONE A
|
|




![Wikipedia: Paxillus atrotomentosus [Fungi]](/images/wikipedia.png)

