|
|
|
|
Year of first isolation: |
1987 |
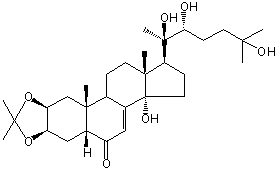
| Formula: | C30H48O7 |
| Molecular weight: | 520 |
| Occurence in plants: |
Rhaponticum carthamoides [Asteraceae] »  ![Wikipedia: Rhaponticum carthamoides [Asteraceae]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
| |
| Canonical SMILES | CC1(OC2CC3C(=O)C=C4C(C3(CC2O1)C)CCC5(C4(CCC5C(C)(C(CCC(C)(C)O)O)O)O)C)C | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@@H]3[C@H]1OC(O3)(C)C)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@](C)([C@@H](CCC(C)(C)O)O)O)O)C)C » 
| | IUPAC Name | (1R,2R,4S,8R,10R,14S,17S,18R)-14-hydroxy-2,6,6,18-tetramethyl-17-[(2R,3R)-2,3,6-trihydroxy-6-methylheptan-2-yl]-5,7-dioxapentacyclo[11.7.0.02,10.04,8.014,18]icos-12-en-11-one | | CAS-RN | | | PubChem CID | 11060392 | InChiKey
[ ChemIDPlus: search ] | OWTQJKQVBMPILJ-VUYJMULXSA-N | | InChI | InChI=1S/C30H48O7/c1-25(2,33)11-10-24(32)29(7,34)23-9-13-30(35)18-14-20(31)19-15-21-22(37-26(3,4)36-21)16-27(19,5)17(18)8-12-28(23,30)6/h14,17,19,21-24,32-35H,8-13,15-16H2,1-7H3/t17-,19-,21+,22-,23-,24+,27+,28+,29+,30+/m0/s1 |
| |
| CI-MS (NH3) m/z | | | EI-MS m/z (relative intensity %) | 520 (M)+ (1), 505 (7), 502 (2), 487 (3), 484 (2), 469 (5), 466 (4), 451 (4), 403 (72), 385 (100), 371 (7), 368 (6), 360 (7), 345 (10), 327 (36), 309 (9),269 (13), 143 (12), 99 (36), 81 (24). | | HR-MS | |
|
|
| CD3OD | | 01 | 38.7 | | 02 | 73.5 | | 03 | 73.2 | | 04 | 27.7 | | 05 | 52.5 | | 06 | 205.6 | | 07 | 121.8 | | 08 | 167.2 | | 09 | 35.7 | | 10 | 38.9 | | 11 | 21.6 | | 12 | 32.5 | | 13 | * | | 14 | 85.2 | | 15 | 31.7 | | 16 | 21.5 | | 17 | 50.5 | | 18 | 18.0 | | 19 | 24.0 | | 20 | 77.9 | | 21 | 21.0 | | 22 | 78.4 | | 23 | 27.3 | | 24 | 42.9 | | 25 | 71.3 | | 26 | 29.7 | | 27 | 28.9 | | 28 | | | 29 | | | iPr- (2,3) | 109.5, 28.8 26.6 |
|
| C5D5N | | 01-Ha | | | 01-He | | | 02-Ha | | | 03-He | | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | 5.99 | | 09-Ha | | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 14-H | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 1.04 (s ) | | 19-Me | 0.88 (s ) | | 21-Me | 1.42 (s ) | | 22-H | | | 23-H | | | 24-Ha | | | 24-Hb | | | 25-H | | | 26-Me | 1.23 (s ) | | 27-Me | 1.23 (s ) | | Other | 1.23 and 1.42 |
| Me2CO-d6 | | 01-Ha | | | 01-He | | | 02-H | 4.23 (m ) | | 02-Ha | | | 03-H | 4.24 (m ) | | 03-He | | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | 5.70 (d, 2.7) | | 09-H | 2.96 (ddd, 11.6, 7.0, 2.7) | | 09-Ha | | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 14-H | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | 2.45 (t, 9.0) | | 18-Me | 0.91 (s ) | | 19-Me | 0.96 (s ) | | 21-Me | 1.19 (s ) | | 22-H | 3.36 (br d, 10.3, <2) | | 23-H | | | 24-Ha | | | 24-Hb | | | 25-H | | | 26-Me | 1.18 (s ) | | 27-Me | 1.17 (s ) | | iPr- (2,3) | 1.42 (s ), 1.26 (s ) | | Other | |
|
| |
| M.P. | 232-233 °C | | [α]D20 | 56.4 ? 2 ° (c 0.9 ; MeOH) | | IR (KBr) ν max (cm-1) | 3460-3500 (OH), 1643 (cyclohexenone) | | UV (EtOH) λ max (log ε) | 244 (4.08) |
| |
| |
Drosophila melanogaster BII cell assay: EC50 = 1.5 x 10-7M |
| |
| First isolation | BALTAYEV, U.A. et al. (1987) Khim. Prir. Soedin. 681-684 |
 | | General | PIS, J. et al. (1994) Phytochemistry 37, 707-711 |
 | | Bioactivities | DINAN, L. et al. (2005) In: Comprehensive Molecular Insect Science (eds. L. I. Gilbert, C. Iatrou, S. Gill), Elsevier, Oxford, III, 197-242 |
 |
| |
Permanent link to this datasheet: 20-HYDROXYECDYSONE 2,3-MONOACETONIDE
|
|




![Wikipedia: Rhaponticum carthamoides [Asteraceae]](/images/wikipedia.png)

