|
|
|
|
Year of first isolation: |
1987 |
| Formula: | C30H48O7 |
| Molecular weight: | 520 |
| Occurence in plants: |
Rhaponticum carthamoides [Asteraceae] »  ![Wikipedia: Rhaponticum carthamoides [Asteraceae]](/images/wikipedia.png)
Vitex strickeri [Lamiaceae (alt. Labiatae)] »  ![Wikipedia: Vitex strickeri [Lamiaceae (alt. Labiatae)]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
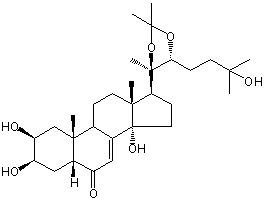
| |
| Canonical SMILES | CC1(OC(C(O1)(C)C2CCC3(C2(CCC4C3=CC(=O)C5C4(CC(C(C5)O)O)C)C)O)CCC(C)(C)O)C | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@]1(C)[C@@H](CCC(C)(C)O)OC(O1)(C)C)O)C)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-17-[(4R,5R)-5-(3-hydroxy-3-methylbutyl)-2,2,4-trimethyl-1,3-dioxolan-4-yl]-10,13-dimethyl-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | 22798-96-5 | | PubChem CID | 11060391 | InChiKey
[ ChemIDPlus: search ] | GXNNYSDWRVKVJY-VUYJMULXSA-N | | InChI | InChI=1S/C30H48O7/c1-25(2,34)11-10-24-29(7,37-26(3,4)36-24)23-9-13-30(35)18-14-20(31)19-15-21(32)22(33)16-27(19,5)17(18)8-12-28(23,30)6/h14,17,19,21-24,32-35H,8-13,15-16H2,1-7H3/t17-,19-,21+,22-,23-,24+,27+,28+,29+,30+/m0/s1 |
| |
| CI-MS (NH3) m/z | | | EI-MS m/z (relative intensity %) | 520 (M)+, 505 (2), 502 (1), 487 (4), 469 (5), 445 (4), 427 (29), 409 (13), 363 (100), 353 (15), 345 (26), 329 (10), 327 (9), 320 (4), 300 (32), 201 (13), 143 (11), 99 (19), 81 (26). | | HR-MS | |
|
|
| CD3OD | | 01 | 37.4 | | 02 | 68.7 | | 03 | 68.5 | | 04 | 32.9 | | 05 | 51.8 | | 06 | 206.5 | | 07 | 122.1 | | 08 | 167.7 | | 09 | 35.1 | | 10 | 39.2 | | 11 | 21.8 | | 12 | 32.3 | | 13 | * | | 14 | 85.3 | | 15 | 31.7 | | 16 | 22.4 | | 17 | 50.5 | | 18 | 17.7 | | 19 | 24.5 | | 20 | 85.9 | | 21 | 22.6 | | 22 | 83.3 | | 23 | 24.7 | | 24 | 42.2 | | 25 | 71.1 | | 26 | 29.5 | | 27 | 29.3 | | iPr-(20,22) | 108.0, 29.0, 27.2 |
|
| Me2CO-d6 | | 01-Ha | | | 01-He | | | 02-Ha | 3.82 m | | 03-He | 3.90 m | | 04-Ha | | | 04-He | | | 05-H | 2.33 (dd, 11.5, 6.0) | | 07-H | 5.72 (d, 2.7) | | 09-Ha | 3.16 (ddd, 11.7, 7.0, 2.7) | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | 2.37 (dd, 9.5, 8.0) | | 18-Me | 0.83 (s) | | 19-Me | 0.93 (s) | | 21-Me | 1.18 (s) | | 22-H | 3.48 (t, 3.0) | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.18 (s) | | 27-Me | 1.17 (s) | | iPr-(20,22) | 1.36 (s), 1.30 (s) |
| C5D5N (/TMS) | | 01-Ha | 1.89 | | 01-He | 3.42 (dd, 5, 12) | | 02-Ha | 3.95 (m, w1/2 = 20) | | 03-He | 4.22 (s, w1/2 = 20) | | 04-Ha | 1.76 | | 04-He | 2.02 | | 05-H | 3.00 (dd, 5, 12) | | 07-H | 6.25 | | 09-Ha | 3.55 (m, w1/2 = 27) | | 11-Ha | 1.72 | | 11-He | 1.88 | | 12-Ha | 2.50 (ddd, 5,10,10) | | 12-He | 2.10 | | 15-Ha | 1.90 | | 15-Hb | 2.10 | | 16-Ha | 2.24 | | 16-Hb | 2.12 | | 17-H | 2.77 | | 18-Me | 1.02 (s ) | | 19-Me | 1.34 (s ) | | 21-Me | 1.06 (s ) | | 22-H | 4.15 (m, w1/2 = 25) | | 23-Ha | 1.90 | | 23-Hb | 2.16 | | 24-Ha | 2.10 | | 24-Hb | 1.74 | | 26-Me | 1.33 (s ) | | 27-Me | 1.37 (s ) | | iPr- 20,22 | 1.55, 1.46 |
|
| |
| M.P. | 227-229 °C | | [α]D20 | + 60.1 ? 2 ° (c 1.3 ; MeOH) | | IR (KBr) ν max (cm-1) | 3400-3465 (OH), 1660 (cyclohexenone) | | UV (EtOH) λ max (log ε) | 243 (4.01) |
| |
| HPTLC | | | TLC | NP-TLC on silica gel Rf 0.09 (CHCl3-EtOH 9:1) | | RLCC | solvent system H2O-EtOAc-n-PrOH (6:6:1) | | GLC | | | Recycle HPLC | column Asahipack GS-320 (50 x 2 cm i.d.) solvent MeOH, flow-rate 3.5 ml.min-1. |
| |
Drosophila melanogaster BII cell assay: EC50 = 3.0 x 10-7M |
| |
| First isolation | BALTAYEV, U.A. et al. (1987) Khim. Prir. Soedin. 681-684 |
 | | General | ZHANG, M. et al. (1992) Phytochemistry 31, 247-250 |
 | | Identification | PIS, J. et al. (1994) Phytochemistry 37, 707-711 |
 | | General | SUKSAMRARN, A. et al. (1995) Tetrahedron 51, 10633-10650 |
 | | Bioactivities | DINAN, L. et al. (2005) In: Comprehensive Molecular Insect Science (eds. L. I. Gilbert, C. Iatrou, S. Gill), Elsevier, Oxford, III, 197-242 |
 |
| |
Permanent link to this datasheet: 20-HYDROXYECDYSONE 20,22-MONOACETONIDE
|
|




![Wikipedia: Rhaponticum carthamoides [Asteraceae]](/images/wikipedia.png)

