|
|
|
|
Year of first isolation: |
1982 |
| Formula: | C27H45O10P |
| Molecular weight: | 560 |
| Occurence in plants: |
|
|
| Occurence in animals: |
Schistocerca gregaria [Orthoptera] »  ![Wikipedia: Schistocerca gregaria [Orthoptera]](/images/wikipedia.png)
Locusta migratoria [Orthoptera] »  ![Wikipedia: Locusta migratoria [Orthoptera]](/images/wikipedia.png)
Bombyx mori [Bombycidae] »  ![Wikipedia: Bombyx mori [Bombycidae]](/images/wikipedia.png)
|
|
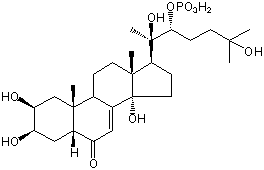
| |
| Canonical SMILES | CC12CCC3C(=CC(=O)C4C3(CC(C(C4)O)O)C)C1(CCC2C(C)(C(CCC(C)(C)O)OP(=O)(O)O)O)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@](C)([C@@H](CCC(C)(C)O)OP(=O)([O-])[O-])O)O)C)C » 
| | IUPAC Name | [(2R,3R)-2,6-dihydroxy-6-methyl-2-[(2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-6-oxo-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-17-yl]heptan-3-yl] dihydrogen phosphate | | CAS-RN | 86577-97-1 | | PubChem CID | 21122089 | InChiKey
[ ChemIDPlus: search ] | VQMWDPXOYFYYKP-YPVLXUMRSA-N | | InChI | InChI=1S/C27H45O10P/c1-23(2,31)9-8-22(37-38(34,35)36)26(5,32)21-7-11-27(33)16-12-18(28)17-13-19(29)20(30)14-24(17,3)15(16)6-10-25(21,27)4/h12,15,17,19-22,29-33H,6-11,13-14H2,1-5H3,(H2,34,35,36)/t15-,17-,19+,20-,21-,22+,24+,25+,26+,27+/m0/s1 |
| |
| FAB-MS m/z | 581 (M-H+Na)-, 559 (M-H)-, 541 (M-H-18)-, 97, 79 EI m/z (direct introduction on gold support): 524 (M-2x18)+, (480), (462), 444, 416, 408 | | HR-MS | |
|
|
| CD3OD | | 01 | | | 02 | 68.7 (d ) | | 03 | 68.6 (d ) | | 04 | | | 05 | | | 06 | | | 07 | | | 08 | | | 09 | | | 10 | | | 11 | | | 12 | | | 13 | | | 14 | 85.3 (s ) | | 15 | | | 16 | | | 17 | | | 18 | | | 19 | | | 20 | 78.5 (s ) | | 21 | | | 22 | 82.9 (d ) | | 23 | | | 24 | | | 25 | 71.4 (s ) | | 26 | | | 27 | |
|
| CD3OD | | 01-Ha | | | 01-He | | | 02-Ha | 3.84 (m, w1/2=22) | | 03-He | 3.95 (m, w1/2=8) | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | 5.80 (d, 2) | | 09-Ha | | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 14-H | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 0.89 (s ) | | 19-Me | 0.96 (s ) | | 21-Me | 1.26 (s ) | | 22-H | 4.07 (m, w1/2=22) | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 25-H | | | 26-Me | 1.18 (s ) |
| D2O | | 01-Ha | | | 01-He | | | 02-Ha | | | 03-He | | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | | | 09-Ha | | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 14-H | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 0.92 | | 19-Me | 0.98 | | 21-Me | 1.32 | | 22-H | | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 25-H | | | 26-Me | 1.21 |
|
| |
| M.P. | | | [α]D20 | | | IR (KBr) ν max (cm-1) | | | UV (EtOH) λ max (log ε) | |
| |
| HPTLC | | | TLC | | | GLC | | | HPLC | Ion-exchange HPLC : Retention volume 32 ml [column Whatman Partisil-SAX 25 cm long, 4.6 mm i.d., eluted with 0.1 M ammonium acetate, flow-rate 2 ml.min-1].Ion-suppression RP-HPLC, Retention volume 31 ml [column Whatman Partisil-ODS3 25 cm long, 4.6 mm i.d., eluted with MeOH in 20mM sodium citrate, pH 6.5, gradient 1:9 v/v to 7:3 v/v over 30 min, flow-rate 2 ml.min-1]. |
| |
| |
| First isolation | TSOUPRAS, G. et al. (1982) Steroids 40, 551-560 |
 | | General | ISAAC, R.E. et al. (1983) Biochem. J. 213, 533-541 |
 | | General | HETRU, C. et al. (1985) Methods Enzymol. 111, 411-419 |
 | | General | DIEHL, P.A. et al. (1985) Methods Enzymol. 111, 377-410 |
 | | General | OHNISHI, E. et al. (1989) Insect Biochem. 19, 95-101 |
 |
| |
Permanent link to this datasheet: 20-HYDROXYECDYSONE 22-PHOSPHATE
|
|




![Wikipedia: Schistocerca gregaria [Orthoptera]](/images/wikipedia.png)

