|
|
|
|
Year of first isolation: |
1986 |
| Formula: | C45H78O7 |
| Molecular weight: | 730 |
| Occurence in plants: |
|
|
| Occurence in animals: |
Boophilus microplus [Ixodidae] »  ![Wikipedia: Boophilus microplus [Ixodidae]](/images/wikipedia.png)
|
|
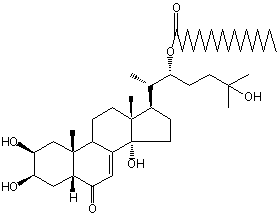
| |
| Canonical SMILES | CCCCCCCCCCCCCCCCCC(=O)OC(CCC(C)(C)O)C(C)C1CCC2(C1(CCC3C2=CC(=O)C4C3(CC(C(C4)O)O)C)C)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@H](C)[C@@H](CCC(C)(C)O)OC(=O)CCCCCCCCCCCCCCCCC)O)C)C » 
| | IUPAC Name | [(2S)-6-hydroxy-6-methyl-2-[(2S,3R,5R,9R,10R,13R,14S,17R)-2,3,14-trihydroxy-10,13-dimethyl-6-oxo-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-17-yl]heptan-3-yl] octadecanoate | | CAS-RN | | | PubChem CID | 66869631 | InChiKey
[ ChemIDPlus: search ] | BQYQZRSEFQCDOC-AVBLRRGPSA-N | | InChI | InChI=1S/C45H78O7/c1-7-8-9-10-11-12-13-14-15-16-17-18-19-20-21-22-41(49)52-40(25-26-42(3,4)50)32(2)33-24-28-45(51)35-29-37(46)36-30-38(47)39(48)31-43(36,5)34(35)23-27-44(33,45)6/h29,32-34,36,38-40,47-48,50-51H,7-28,30-31H2,1-6H3/t32-,33+,34-,36-,38+,39-,40?,43+,44+,45+/m0/s1 |
| |
| CI-MS (NH3) m/z | | | EI-MS m/z (relative intensity %) | | | FAB-MS (matrix tetraethyleneglycol TEG) m/z (relative intensity %) | 925 (M+H+ TEG)+ (1), 731 (M+H)+ (7), 713 (40), 695 (5), 447 (11), 429 (100), 411 (45), 393 (10). | | HR-MS | |
|
|
| C5D5N | | 01 | 38.7 | | 02 | 68.1 | | 03 | 68.0 | | 04 | 32.5 | | 05 | 51.4 | | 06 | 203.0 | | 07 | 121.7 | | 08 | 165.3 | | 09 | 35.0 | | 10 | 38.0 | | 11 | 21.1 | | 12 | 31.5 | | 13 | 47.6 | | 14 | 83.9 | | 15 | 31.9 | | 16 | 26.4 | | 17 | 48.3 | | 18 | 15.8 | | 19 | 24.5 | | 20 | 39.9 | | 21 | 14.3 | | 22 | 77.7 | | 23 | 25.6 | | 24 | 41.6 | | 25 | 69.3 | | 26 | 30.0 | | 27 | 30.3 | | CH2 | 23.0, 29.5, 29.6, 29.8, 29.87, | | CH2- | 29.93, 30.0, 32.1, 34.6 | | COOR | 173.6 | | Me | 14.5 |
|
| CDCl3OD | | 01-Ha | | | 01-He | | | 02-Ha | 3.92 (m, w1/2=22) | | 03-He | 4.06 (m, w1/2=10) | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | 5.855 (d, 2) | | 09-Ha | | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 14-H | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 0.677 (s ) | | 19-Me | 0.993 (s ) | | 21-Me | 0.949 (d, 7) | | 22-H | 4.89 (m, w1/2=17) | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 25-H | | | 26-Me | 1.232 (s ) | | 27-Me | 1.232 (s ) |
|
| |
| M.P. | | | [α]D20 | | | IR (KBr) ν max (cm-1) | | | UV (EtOH) λ max (log ε) | |
| |
| HPTLC | | | TLC | Rf 0.14 (silica gel, CHCl3-MeOH 9:1); Rf 0.25 (C8 bonded silica gel, MeOH-H2O 19:1), other solvent systems also described for C18, C2 and CN bonded silica gel. | | GLC | (of fatty acid methyl ester) | | HPLC | 1- Retention time 24.5 min [Partisil 5 (Whatman), 250 mm x 4.6 mm i.d., solvent dichloroethane-iPrOH-H2O 125:24:1 v/v/v, flow-rate 1 ml.min-1]. 2-Retention time 27.5 min [ Ultrasphere-ODS (Beckman) 250 mm x 4.6 mm i.d., solvent linear gradient (30 min) of MeOH in H2O from 9:1 to 1:0 v/v, flow-rate 1 ml.min-1] |
| |
| |
| General | WIGGLESWORTH, K.P. et al. (1985) Arch. Insect Biochem. Physiol. 2, 39-54 |
 | | General | HOFFMANN, K.H. et al. (1985) Life Sci. 37, 185-192 |
 | | First isolation | CROSBY, T. et al. (1986) Biochem. J. 240, 131-138 |
 | | General | ROBINSON, P.D. et al. (1987) Physiol. Entomol. 12, 321-330 |
 | | General | DINAN, L. et al. (1987) J. Chromatogr. 411, 379-392 |
 | | General | DINAN, L. et al. (1988) J. Steroid Biochem. 31, 237-245 |
 | | General | DINAN, L. et al. (1988) J. Chromatogr. 436, 279-288 |
 |
| |
Permanent link to this datasheet: ECDYSONE 22-STEARATE
|
|




![Wikipedia: Boophilus microplus [Ixodidae]](/images/wikipedia.png)

