|
|
|
|
Year of first isolation: |
1973 |
| Formula: | C27H40O7 |
| Molecular weight: | 476 |
| Occurence in plants: |
Ipomoea calonyction [Convolvulaceae] »  ![Wikipedia: Ipomoea calonyction [Convolvulaceae]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
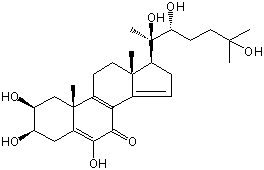
| |
| Canonical SMILES | CC12CCC3=C(C1=CCC2C(C)(C(CCC(C)(C)O)O)O)C(=O)C(=C4C3(CC(C(C4)O)O)C)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2(C(=C(C(=O)C3=C2CC[C@]2(C3=CC[C@@H]2[C@](C)([C@@H](CCC(C)(C)O)O)O)C)O)C[C@H]([C@H]1O)O)C » 
| | IUPAC Name | (2S,3R,10R,13R,17S)-2,3,6-trihydroxy-10,13-dimethyl-17-[(2R,3R)-2,3,6-trihydroxy-6-methylheptan-2-yl]-1,2,3,4,11,12,16,17-octahydrocyclopenta[a]phenanthren-7-one | | CAS-RN | 51787-31-6 | | PubChem CID | 101281312 | InChiKey
[ ChemIDPlus: search ] | XAKKXZNYERXAIY-HTYOSDJUSA-N | | InChI | InChI=1S/C27H40O7/c1-24(2,33)10-9-20(30)27(5,34)19-7-6-14-21-15(8-11-25(14,19)3)26(4)13-18(29)17(28)12-16(26)22(31)23(21)32/h6,17-20,28-31,33-34H,7-13H2,1-5H3/t17-,18+,19+,20-,25+,26-,27-/m1/s1 |
| |
| EI-MS m/z (relative intensity %) | 476 (14.4), 458 (32.4), 443 (18.0), 442 (16.2), 440 (38.8), 425 (22.8), 424 (9.2), 422 (18.2), 407 (12.8), 406 (5.9), 359 (56.5), 343 (48), 342 (100), 341 (59.1), 327 (93.1), 325 (41.0), 323 (39.2), 315 (64.2), 314 (80.5), 298 (75.8), 296 (40.5), 161, 143, 125, 107, 99, 81, 69, 55 | | HR-MS | 476.2774 (M)+ (14.4%), calc. 476.2774 for C27H40O7) | | CI/D | 477 (M+H)+, 459, 441, 427, 425, 423, 376, 361, 359, 345, 343, 341 |
|
|
| DMSO-d6 | | 01 | | | 02 | | | 03 | | | 04 | | | 05 | | | 06 | | | 07 | | | 08 | | | 09 | | | 10 | | | 11 | | | 12 | | | 13 | | | 14 | 84.2 | | 15 | | | 16 | | | 17 | | | 18 | | | 19 | | | 20 | 74.9 | | 21 | | | 22 | 76.4 | | 23 | 20.0 | | 24 | | | 25 | 68.6 | | 26 | 29.8 | | 27 | 29.8 |
|
| C5D5N | | 01-Ha | | | 01-Hb | | | 02-Ha | | | 03-He | | | 04-Ha | | | 04-He | | | 07-H | | | 09-Ha | | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-H | 7.41 (m, w1/2 = 4) | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 1.40 (s ) | | 19-Me | 1.72 (s ) | | 21-Me | 1.56 (s ) | | 22-H | | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.42 (s ) | | 27-Me | 1.42 (s ) |
| DMSO-d6 | | 01-Ha | | | 01-Hb | | | 02-Ha | | | 03-He | | | 04-Ha | | | 04-He | | | 07-H | | | 09-Ha | | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-H | 6.78 | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 1.00 | | 19-Me | 1.41 | | 21-Me | 1.16 | | 22-H | | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.05 | | 27-Me | 1.07 |
|
| |
| M.P. | 234-235 °C | | [α]D20 | +76.8 ° (c 1; MeOH) | | IR (KBr) ν max (cm-1) | 3380, 3350 (OH), 1638, 1620, 845 (C=C, C=O conj.) | | UV (EtOH) λ max (log ε) | 222 (4.32), 233(4.13), 294 (3.89) |
| |
| HPTLC | | | TLC | NP-TLC on silica gel Rf 0.42 (CHCl3-EtOH 4:1); RP-TLC on paraffin coated silica gel Rf 0.32 (MeOH-H2O 50:50); RP-TLC on C18-bonded silica gel Rf 0.28 (MeOH-H2O 65:35) | | GLC | Ret 10.5 min on 1.5% OV 101 (0.9 m x 4.5 mm i.d.) at 285°C after silylation at 140°C for 60 h. | | HPLC | |
| |
Drosophila melanogaster BII cell assay: inactive |
| |
| First isolation | CANONICA, L. et al. (1973) J. Chem. Soc., Chem. Commun., 737-738 |
 | | General | CANONICA, L. et al. (1975) Phytochemistry 14, 525-527 |
 | | General | CANONICA, L. et al. (1977) Gazz. Chim. Ital. 107, 229-235 |
 | | General | WILSON, I.D. et al. (1985) J. Chromatogr. 318, 373-377 |
 | | General | BIELBY, C.R. et al. (1986) J. Chromatogr. 351, 57-64 |
 | | General | SUKSAMRARN, A. et al. (1994) Tetrahedron 35, 4445-4448 |
 | | Synthesis | SUKSAMRARN, A. et al. (1997) Phytochemistry 45, 1149-1152 |
 | | Bioactivities | DINAN, L. et al. (1999) J. Computer-aided Mol. Design 13, 185-207 |
 |
| |
Permanent link to this datasheet: CALONYSTERONE
|
|




![Wikipedia: Ipomoea calonyction [Convolvulaceae]](/images/wikipedia.png)

