|
|
|
|
Year of first isolation: |
1995 |
| Formula: | C29H42O9 |
| Molecular weight: | 534 |
| Occurence in plants: |
Ajuga reptans var. atropurpurea [Lamiaceae (alt. Labiatae)] »  ![Wikipedia: Ajuga reptans var. atropurpurea [Lamiaceae (alt. Labiatae)]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
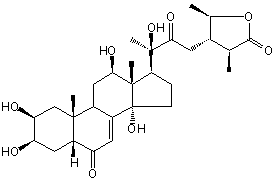
| |
| Canonical SMILES | CC1C(C(OC1=O)C)C=C(C(C)(C2CCC3(C2(C(CC4C3=CC(=O)C5C4(CC(C(C5)O)O)C)O)C)O)O)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2C[C@H]([C@]2([C@]1(CC[C@@H]2[C@](C)(C(=O)C[C@H]1[C@@H](C(=O)O[C@@H]1C)C)O)O)C)O)C » 
| | IUPAC Name | (3S,4S,5R)-4-[(Z,3R)-2,3-dihydroxy-3-[(2S,3R,5R,9R,10R,13S,14R,17S)-2,3,12,14-tetrahydroxy-10,13-dimethyl-6-oxo-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-17-yl]but-1-enyl]-3,5-dimethyloxolan-2-one | | CAS-RN | | | PubChem CID | 101699886 | InChiKey
[ ChemIDPlus: search ] | JQEDSZYKGHQUGZ-AKPXYYRYSA-N | | InChI | InChI=1S/C29H42O9/c1-13-15(14(2)38-25(13)35)8-24(34)28(5,36)22-6-7-29(37)17-9-19(30)18-10-20(31)21(32)12-26(18,3)16(17)11-23(33)27(22,29)4/h8-9,13-16,18,20-23,31-34,36-37H,6-7,10-12H2,1-5H3/b24-8-/t13-,14+,15-,16-,18-,20+,21-,22-,23?,26+,27-,28+,29+/m0/s1 |
| |
| MS (thermospray) m/z (negative ions) | 579 (M+HCOO)-, 561 (M+HCOO-H2O)- | | HR-MS | |
|
|
| C5D5N | | 01 | 37.9 | | 02 | 68.0 | | 03 | 67.9 | | 04 | 32.4 | | 05 | 51.2 | | 06 | 203.3 | | 07 | 122.3 | | 08 | 163.1 | | 09 | 34.5 | | 10 | 38.8 | | 11 | 29.7 | | 12 | 71.0 | | 13 | 51.8 | | 14 | 85.9 | | 15 | 31.6 | | 16 | 23.0 | | 17 | 59.3 | | 18 | 12.1 | | 19 | 24.3 | | 20 | 79.0 | | 21 | 28.8 | | 22 | 218.4 | | 23 | 42.0 | | 24 | 45.8 | | 25 | 42.2 | | 26 | 178.6 | | 27 | 14.7 | | 28 | 79.8 | | 29 | 20.2 |
|
| C5D5N | | 01-Ha | 2.11 | | 01-He | 2.02 | | 02-Ha | 4.16 (m, w1/2=24) | | 03-He | 4.22 | | 04-Ha | 2.02 | | 04-He | 1.74 (t, 14) | | 05-H | 3.04 (dd, 13.0, 3.5) | | 07-H | 6.30 (d, 2) | | 09-H | 3.78 (m, w1/2=26) | | 11-Ha | 2.45 | | 11-He | 1.92 | | 12-He | 5.08 | | 15-Ha | 2.19 | | 15-Hb | 1.95 | | 16-Ha | 2.83 (m) | | 16-Hb | 2.25 | | 17-H | 3.42 (t, 9.5) | | 18-Me | 0.84 (s) | | 19-Me | 1.08 (s) | | 21-Me | 1.58 (s) | | 22-H | ? | | 23-Ha | 3.57 (dd, 19.0, 6.0) | | 23-Hb | 3.31 (dd, 19.0, 6.0) | | 24-Ha | 2.40 | | 25-H | 2.53 (dq, 11.0, 7.0) | | 27-Me | 1.31 (d, 7) | | 28-H | 4.24 (dq, 8.5, 6.0) | | 29-Me | 1.41 (d, 6.5) |
|
| |
| M.P. | | | [α]D20 | | | IR (KBr) ν max (cm-1) | 3405, 1757, 1708, 1658 | | UV (EtOH) λ max (log ε) | |
| |
| HPTLC | | | TLC | | | GLC | | | HPLC | RP-HPLC, column Spherisorb ODS-2, 10 ?m, 300 x 7.8 mm, flow-rate 3 ml.min-1, solvent iPrOH-H2O 1:5.6, temp. 23°C, Ret. 60.0 min (20E 17.2 min). |
| |
Drosophila melanogaster BII cell assay: EC50 = 1.3 x 10-6M |
| |
| |
Permanent link to this datasheet: 22-DEHYDRO-12-HYDROXYCYASTERONE
|
|




![Wikipedia: Ajuga reptans var. atropurpurea [Lamiaceae (alt. Labiatae)]](/images/wikipedia.png)

