|
|
|
|
Year of first isolation: |
2025 |
| Formula: | C32H50O8 |
| Molecular weight: | 562 |
| Occurence in plants: |
Dianthus superbus L. (Caryophyllaceae) [Fungi] » 
|
| Occurence in animals: |
|
|
|
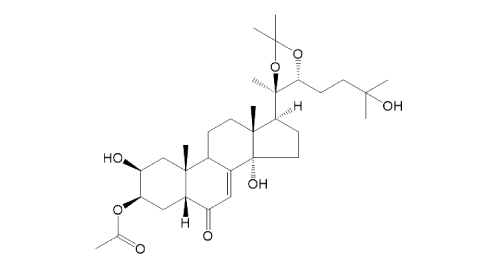
| |
Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C[C@@]12[C@@](C(C=C3C2CC[C@@]4(C)[C@@]3(O)CC[C@]4([H])[C@@]5(C)OC(C)(C)O[C@@H]5CCC(C)(O)C)=O)([H])C[C@@H](OC(C)=O)[C@@H](O)C1 » 
| | IUPAC Name | (2S,3R,5R,10R,13R,14S,17S)-2,14-dihydroxy-17-((4R,5R)-5-(3-hydroxy-3-methylbutyl)-2,2,4-trimethyl-1,3-dioxolan-4-yl)-10,13-dimethyl-6-oxo-2,3,4,5,6,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate | | CAS-RN | | | PubChem CID | | InChiKey
[ ChemIDPlus: search ] | ZDVRRFITQUTQOE-LQNOMGLBSA-N | | InChI | InChI=1S/C32H50O8/c1-18(33)38-24-16-21-22(34)15-20-19(29(21,6)17-23(24)35)9-13-30(7)25(10-14-32(20,30)37)31(8)26(11-12-27(2,3)36)39-28(4,5)40-31/h15,19,21,23-26,35-37H,9-14,16-17H2,1-8H3/t19?,21-,23-,24+,25-,26+,29+,30+,31+,32+/m0/s1 |
| |
| HRESIMS (positive ion mode) m/z
| [M+Na]+ found 585.3392, calc. for C32H50O8Na+ 585.3398 |
|
|
| CD3OD | | 01 | 38.4 | | 02 | 67.1 | | 03 | 71.8 | | 04 | 30.3 | | 05 | 52.5 | | 06 | 205.4 | | 07 | 122.0 | | 08 | 168.0 | | 09 | 35.2 | | 10 | 39.1 | | 11 | 21.6 | | 12 | 32.3 | | 13 | 49.1 | | 14 | 85.3 | | 15 | 31.7 | | 16 | 22.4 | | 17 | 50.5 | | 18 | 17.7 | | 19 | 24.4 | | 20 | 85.8 | | 21 | 22.6 | | 22 | 83.3 | | 23 | 24.7 | | 24 | 42.2 | | 25 | 71.1 | | 26 | 29.0 | | 27 | 29.4 | | [1]' | 172.5 | | [1]'' | 108.0 | | [2]' | 21.1 | | [2]'' | 27.2 | | [3]'' | 29.5 |
|
| CD3OD | | 01-Ha | 1.91 (m) | | 01-Hb | 1.42 (m) | | 02-Ha | 3.97 (dt, 12.3,4.0)) | | 03-He | 5.5 (m) | | 04-Ha | 1.76 (m) | | 04-Hb | - | | 05-H | 2.22 (dd, 12.1, 5.4) | | 07-H | 5.83 (d, 2.5) | | 09-H | 3.17 (td, 7.8, 3.8) | | 11-Ha | 1.81 (m) | | 11-Hb | 1.68 (m) | | 12-Ha | 2.12 (m) | | 12-Hb | 1.86 (m) | | 15-Ha | 1.93 (m) | | 15-Hb | 1.61 (m) | | 16-Ha | 1.96 (m) | | 16-Hb | 1.91 (m) | | 17-H | 2.32 (m) | | 18-Me | 0.83 (s) | | 19-Me | 1.00 (s) | | 21-Me | 1.18 (s) | | 22-H | 3.68 (m) | | 23-Ha | 1.56 (m) | | 23-Hb | - | | 24-Ha | 1.73 (m) | | 24-Hb | 1.49 (m) | | 26-Me | 1.20 (s) | | 27-Me | 1.21 (s) | | [2]''-Me | 1.32 (s) | | [2]'-Me | 2.12 (s) | | [3]''-Me | 1.39 (s) |
|
| |
| M.P. | — °C ; | | [α]D20 | - ° (c ; MeOH) | | IR (KBr) ν max (cm-1) | | | UV (MeOH) λ max (log ε) | - nm (-) ; |
| |
| |
| |
| |
Permanent link to this datasheet: 3-O-ACETYL-20-HYDROXYECDYSONE-20,22-MONOACETONIDE
|
|





