|
|
|
|
Year of first isolation: |
1968 |
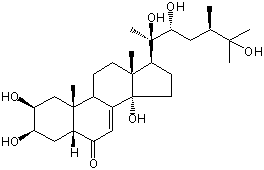
| Formula: | C28H46O7 |
| Molecular weight: | 494 |
| Occurence in plants: |
Podocarpus macrophyllus [Podocarpaceae] »  ![Wikipedia: Podocarpus macrophyllus [Podocarpaceae]](/images/wikipedia.png)
Podocarpus elatus [Podocarpaceae] »  ![Wikipedia: Podocarpus elatus [Podocarpaceae]](/images/wikipedia.png)
Lychnis flos-cuculi [Caryophyllaceae] »  ![Wikipedia: Lychnis flos-cuculi [Caryophyllaceae]](/images/wikipedia.png)
Palisota schweinfurthii [Commelinaceae] »  ![Wikipedia: Palisota schweinfurthii [Commelinaceae]](/images/wikipedia.png)
Leuzea carthamoides [Asteraceae] »  ![Wikipedia: Leuzea carthamoides [Asteraceae]](/images/wikipedia.png)
|
| Occurence in animals: |
Oncopeltus fasciatus [Hemiptera] »  ![Wikipedia: Oncopeltus fasciatus [Hemiptera]](/images/wikipedia.png)
Drosophila melanogaster [Diptera] »  ![Wikipedia: Drosophila melanogaster [Diptera]](/images/wikipedia.png)
Apis mellifera [Apidae] »  ![Wikipedia: Apis mellifera [Apidae]](/images/wikipedia.png)
Callinectes sapidus [Portunidae] »  ![Wikipedia: Callinectes sapidus [Portunidae]](/images/wikipedia.png)
|
|
| |
| Canonical SMILES | CC(CC(C(C)(C1CCC2(C1(CCC3C2=CC(=O)C4C3(CC(C(C4)O)O)C)C)O)O)O)C(C)(C)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@](C)([C@H](O)C[C@H](C(C)(C)O)C)O)O)C)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-17-[(2R,3R,5R)-2,3,6-trihydroxy-5,6-dimethylheptan-2-yl]-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | 20137-14-8 | | PubChem CID | 12312690 | InChiKey
[ ChemIDPlus: search ] | IJRBORPEVKCEQD-JMQWOFAPSA-N | | InChI | InChI=1S/C28H46O7/c1-15(24(2,3)33)11-23(32)27(6,34)22-8-10-28(35)17-12-19(29)18-13-20(30)21(31)14-25(18,4)16(17)7-9-26(22,28)5/h12,15-16,18,20-23,30-35H,7-11,13-14H2,1-6H3/t15-,16+,18+,20-,21+,22+,23-,25-,26-,27-,28-/m1/s1 |
| |
| EI-MS m/z (relative intensity %) | 494 (M)+, 363 (100), 345 (78), 131 (24), 113 (59), 95 (30), 70 (100) | | HR-MS | |
|
|
| C5D5N | | 01 | 37.8 | | 02 | 68.0 | | 03 | 68.0 | | 04 | 32.3 | | 05 | 51.3 | | 06 | 203.6 | | 07 | 121.6 | | 08 | 166.1 | | 09 | 34.4 | | 10 | 38.6 | | 11 | 21.1 | | 12 | 31.7 | | 13 | 48.1 | | 14 | 84.2 | | 15 | 32.0 | | 16 | 21.3 | | 17 | 49.9 | | 18 | 17.9 | | 19 | 24.4 | | 20 | 77.0 | | 21 | 21.5 | | 22 | 74.7 | | 23 | 34.4 | | 24 | 41.7 | | 25 | 72.2 | | 26 | 26.3 * | | 27 | 28.2 * | | 28 | 15.4 |
| CD3OD | | 01 | 37.3 | | 02 | 68.5 | | 03 | 68.7 | | 04 | 32.6 | | 05 | 51.7 | | 06 | 206.4 | | 07 | 122.1 | | 08 | 167.9 | | 09 | 35.1 | | 10 | 39.3 | | 11 | 21.5 | | 12 | 31.8 | | 14 | 85.2 | | 15 | 32.8 | | 16 | 21.4 | | 17 | 50.4 | | 18 | 18.1 | | 19 | 24.4 | | 20 | 77.9 | | 21 | 21.0 | | 22 | 75.3 | | 23 | 34.4 | | 24 | 41.5 | | 25 | 73.7 | | 26 | 27.4 | | 27 | 26.1 | | 28 | 14.9 |
|
| D2O | | 01-Ha | | | 01-Hb | | | 02-Ha | 3.99 (m, w1/2=22) | | 03-He | 4.07 (m, w1/2=8) | | 04-Ha | | | 04-He | | | 05-H | 2.34 (t | | 07-H | 5.97 (d, 2) | | 09-Ha | 3.11 (m, w1/2=22) | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | 2.29 (m, t ) | | 18-Me | 0.87 (s ) | | 19-Me | 1.00 (s ) | | 21-Me | 1.23 (s ) | | 22-Hb | 3.56 (d, 10.6) | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.17 (s ) | | 27-Me | 1.20 (s ) | | 28-Me | 0.95 (d, 6.9) |
| Me2CO-d6 | | 01-Ha | | | 01-Hb | | | 02-Ha | 3.82 dt (11.3, 3.8, 3.8) | | 03-He | 3.90 um | | 04-Ha | | | 04-He | | | 05-H | 2.33 dd (10.2, 7.1) | | 07-H | 5.71 d (2.6) | | 09-Ha | 3.16 ddd (10.0, 7.0, 2.6) | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | 2.43 t (9.1) | | 18-Me | 0.92 s | | 19-Me | 0.94 s | | 21-Me | 1.19 s | | 22-Hb | 3.46 br d (11.0, <2) | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.15 s | | 27-Me | 1.11 s | | 28-Me | 0.92 d (6.8) |
|
| |
| M.P. | 263-265°C | | [α]D20 | + 60.3° (dioxane) | | IR (KBr) ν max (cm-1) | 3420, 1655, 1630 | | UV (MeOH) λ max (log ε) | 243 (4.09) |
| |
| HPTLC | | | TLC | NP-TLC silica : Rf 0.21 (EtOAc-MeOH-NH4OH 85:10:5); Rf 0.19 (CH2Cl2-EtOH 85:15)Rf 0.16 (CHCl3-EtOH 4:1); Rf 0.20 (CHCl3-EtOH 4:1); RP-TLC on paraffin-coated silica gel : Rf 0.33 (MeOH-H2O 50:50); RP-TLC on C18-bonded silica gel : Rf 0.40 (MeOH-H2O 65:35) | | GLC | Ret 9.15 min on 1.5% OV101 (0.9m x 4mm i.d.) at 285°C following silylation at 140°C for 60 h. | | NP-HPLC | (Zorbax-Sil) Ret. 12.7 min (solvent CH2Cl2-iPrOH-H2O, 125:40:3) (20E : 16.8 min); | | RP-HPLC | (Spherisorb-ODS2) Ret. 7.3 min (solvent ACN-0.1% TFA in H2O, 23:77) (20E : 5.5 min). |
| |
Drosophila melanogaster BII cell assay: EC50 = 1.3 x 10-8M | Calliphora vicina imaginal disc assay: EC50 = 1.2 x 10-7M | Drosophila melanogaster Kc-H cell assay: EC50 = 2.3 x 10-8M | Calliphora assay: 100% (20-hydroxyecdysone = 100%) |
| |
| First isolation | IMAI, S. et al. (1968) Tetrahedron Lett. 3883-3886 |
 | | General | GALBRAITH, M.N. et al. (1969) J. Chem. Soc., Chem. Commun. 402-403 |
 | | General | FAUX, A. et al. (1969) J. Chem. Soc., Chem. Commun. 175-176 |
 | | Struct. analysis | DANIELI, B. et al. (1974) Chem. Commun., 745-746 |
 | | General | KAPLANIS, J.N. et al. (1975) Science 190, 681-682 |
 | | Bioactivities | CHERBAS, L. et al. (1980) W. Roux Arch. Devel. Biol. 189, 1-15 |
 | | Bioactivities | BERGAMASCO, R. et al. (1980) In: Progress in Ecdysone Research (Ed. J.A. Hoffmann), Elsevier/North Holland Biomed. Press, Amsterdam 299-324 |
 | | General | REDFERN, C.P.F. et al. (1984) Proc. Natl. Acad. Sci. U.S.A. 81, 5643-5647 |
 | | General | FELDLAUFER, M.F. et al. (1986) Insect Biochem. 16, 45-48 |
 | | Bioactivities | CLEMENT, C.Y. et al. (1993) Insect Biochem. Molec. Biol. 23, 187-193 |
 | | Bioactivities | TERENTIOU, P. et al. (1993) Insect Biochem. Molec. Biol. 23, 131-136 |
 | | Identification | PIS, J. et al. (1994) Phytochemistry 37, 707-711 |
 | | General | KUSAMBA, C. et al. (1995) Fitoterapia LXVI, 175-178 |
 |
| |
Permanent link to this datasheet: MAKISTERONE A
|
|




![Wikipedia: Podocarpus macrophyllus [Podocarpaceae]](/images/wikipedia.png)

