|
|
|
|
Year of first isolation: |
1986 |
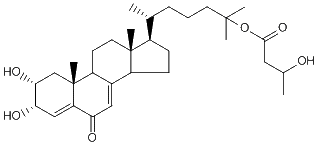
| Formula: | C31H48O6 |
| Molecular weight: | 516 |
| Occurence in plants: |
|
|
| Occurence in animals: |
Diaulula sandiegensis [Mollusca] »  ![Wikipedia: Diaulula sandiegensis [Mollusca]](/images/wikipedia.png)
|
|
| |
| Canonical SMILES | CC(CCCC(C)(C)OC(=O)CC(C)O)C1CCC2C1(CCC3C2=CC(=O)C4=CC(C(CC34C)O)O)C | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2(C(=C[C@@H]([C@@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2(C1CC[C@@H]2[C@H](C)CCCC(C)(C)OC(=O)CC(C)O)C)C » 
C1[C@]2(C(=C[C@@H]([C@@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@H]1CC[C@@H]2[C@H](C)CCCC(C)(C)OC(=O)CC(C)O)C)C » 
C1[C@]2(C(=C[C@@H]([C@@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@@H]1CC[C@@H]2[C@H](C)CCCC(C)(C)OC(=O)CC(C)O)C)C » 
| | IUPAC Name | [6-[(2R,3S,10R,13R,17R)-2,3-dihydroxy-10,13-dimethyl-6-oxo-1,2,3,9,11,12,14,15,16,17-decahydrocyclopenta[a]phenanthren-17-yl]-2-methylheptan-2-yl] 3-hydroxybutanoate | | CAS-RN | | | PubChem CID | 22832540 | InChiKey
[ ChemIDPlus: search ] | HVGPOQMKFKPULV-NTCDBHQHSA-N | | InChI | InChI=1S/C31H48O6/c1-18(8-7-12-29(3,4)37-28(36)14-19(2)32)21-9-10-22-20-15-25(33)24-16-26(34)27(35)17-31(24,6)23(20)11-13-30(21,22)5/h15-16,18-19,21-23,26-27,32,34-35H,7-14,17H2,1-6H3/t18?,19?,21-,22?,23?,26+,27-,30-,31-/m1/s1 |
| |
| CI-MS (NH3) m/z | | | EI-MS m/z (relative intensity %) | | | HR-MS | |
|
|
| CDCl3 | | 01 | | | 02 | 66.7 | | 03 | 64.4 | | 04 | 128.1 | | 05 | 146.2 | | 06 | 188.7 | | 07 | 123.6 | | 08 | 166.9 | | 09 | | | 10 | | | 11 | | | 12 | | | 13 | | | 14 | | | 15 | | | 16 | | | 17 | | | 18 | 12.5 | | 19 | 21.1 | | 20 | | | 21 | 18.7 | | 22 | | | 23 | | | 24 | | | 25 | 83.6 | | 26 | 26.1 | | 27 | 26.2 | | C | 172.4 | | CH | 65.2 | | CH2 | 42.1 | | CH3 | 22.3 |
|
| CDCl3 | | 01-Ha | | | 01-Hb | | | 02-He | 3.90 (dt, 11.5) | | 03-Ha | 4.28 (t, 5) | | 04-H | 6.54 (d, 5) | | 07-H | 5.87 (t, 1) | | 09-Ha | | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 14-H | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 0.65 (s ) | | 19-Me | 1.17 (s ) | | 20-H | | | 21-Me | 0.96 (d, 7) | | 22-Ha | | | 22-Hb | | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.46 (s ) | | 27-Me | 1.46 (s ) | | 29/30-CH2 | 2.34 (dd, 9,16); 2.42 (dd, 4,16) | | 31-CH | 4.15 (m ) | | 32-Me | 1.21 (d, 7) |
|
| |
| M.P. | | | [α]D20 | | | IR (KBr) ν max (cm-1) | 3380 (OH), 1722 (ester), 1660 (cyclohexenone), 1620 (ene) | | UV (EtOH) λ max (log ε) | |
| |
| HPTLC | | | TLC | (silica) Rf 0.18 (EtOAc) | | GLC | | | HPLC | column Whatman magnum ODS-3, solvent MeOH-H2O (7:3) |
| |
| |
| |
Permanent link to this datasheet: DIAULUSTEROL A
|
|




![Wikipedia: Diaulula sandiegensis [Mollusca]](/images/wikipedia.png)

