|
|
|
|
Year of first isolation: |
1970 |
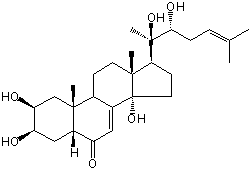
| Formula: | C27H42O6 |
| Molecular weight: | 462 |
| Occurence in plants: |
Stachyurus praecox [Stachyuraceae] »  ![Wikipedia: Stachyurus praecox [Stachyuraceae]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
| |
| Canonical SMILES | CC(=CCC(C(C)(C1CCC2(C1(CCC3C2=CC(=O)C4C3(CC(C(C4)O)O)C)C)O)O)O)C | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@](C)([C@@H](CC=C(C)C)O)O)O)C)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,13R,14S,17S)-17-[(3R)-2,3-dihydroxy-6-methylhept-5-en-2-yl]-2,3,14-trihydroxy-10,13-dimethyl-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | 26362-25-4 | | PubChem CID | 24984907 | InChiKey
[ ChemIDPlus: search ] | WBOQDRZZNALTIW-VUMURMMZSA-N | | InChI | InChI=1S/C27H42O6/c1-15(2)6-7-23(31)26(5,32)22-9-11-27(33)17-12-19(28)18-13-20(29)21(30)14-24(18,3)16(17)8-10-25(22,27)4/h6,12,16,18,20-23,29-33H,7-11,13-14H2,1-5H3/t16-,18-,20+,21-,22-,23+,24+,25+,26?,27+/m0/s1 |
| |
| FAB-MS (relative intensity %) | 463 [M+H]+ (54), 445 (29
463 [M+H]+ (54), 445 (29), 427 (6).
| | CI-MS (NH3) m/z | 480 (MH + NH3)+, 463 (MH)+, 445, 429, 427, 380 (M-C22-C27 + NH3)+, 363, 347, 345 | | EI-MS m/z (relative intensity %) | 462 (M)+, 444 (M-18), 363 (25), 345 (74), 327 (25), 99 (C22-C27; 9), 81 (23), 69 (C23-C27; 49), 43 (100). | | HR-MS | 444.2863 (M-18), calculated 444.2874 |
|
|
| C5D5N | | 01 | | | 02 | | | 03 | | | 04 | | | 05 | | | 06 | | | 07 | | | 08 | | | 09 | | | 10 | | | 11 | | | 12 | | | 13 | | | 14 | | | 15 | | | 16 | | | 17 | | | 18 | | | 19 | | | 20 | | | 21 | | | 22 | | | 23 | | | 24 | | | 25 | | | 26 | | | 27 | |
|
| C5D5N | | 01-Ha | | | 01-Hb | | | 02-Ha | 4.17 (m) | | 03-He | 4.24 (br s) | | 04-Ha | | | 04-He | | | 05-H | 3.02 (dd 13.2, 3.5) | | 07-H | 6.25 (d 2.1) | | 09-Ha | 3.60 (m) | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | 2.95 (t 9.3) | | 18-Me | 1.21 (s) | | 19-Me | 1.07 (s) | | 21-Me | 1.59 (s) | | 22-H | 3.89 (dd 9.7, 1.5) | | 23-Ha | | | 23-Hb | | | 24-H | 5.55 (br t 7) | | 26-Me | 1.59 (s) | | 27-Me | 1.63 (s) |
|
| |
| M.P. | 225-227 °C | | [α]D20 | + 43 ° (c ; dioxane) | | IR (KBr) ν max (cm-1) | 3414, 2926, 1647, 1445, 1382, 1116, 1056, 875 | | UV (EtOH) λ max (log ε) | 242 (4.02) |
| |
| |
Drosophila melanogaster BII cell assay: EC50 = 1.4 x 10-8M | Sarcophaga assay: highly active | Drosophila melanogaster BII cell assay: EC50 = 7.5 x 10-9M |
| |
| First isolation | IMAI, S. et al. (1970) J. Chem. Soc., Chem. Commun. 352-353 |
 | | Bioactivities | BERGAMASCO, R. et al. (1980) In: Progress in Ecdysone Research (Ed. J.A. Hoffmann), Elsevier/North Holland Biomed. Press, Amsterdam 299-324 |
 | | General | PIS, J. et al. (1995) Steroids 60, 188-194 |
 | | Bioactivities | DINAN, L. et al. (1999) J. Computer-aided Mol. Design 13, 185-207 |
 | | Bioactivities | DINAN, L. et al. (2005) In: Comprehensive Molecular Insect Science (eds. L. I. Gilbert, C. Iatrou, S. Gill), Elsevier, Oxford, III, 197-242 |
 | | Synthesis | YINGYONGNARONGKUL, B.-E. et al. (2005) Steroids 70, 636-644 |
 |
| |
Permanent link to this datasheet: STACHYSTERONE C
|
|




![Wikipedia: Stachyurus praecox [Stachyuraceae]](/images/wikipedia.png)

