|
|
|
|
Year of first isolation: |
1995 |
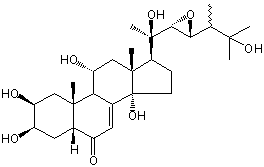
| Formula: | C28H44O8 |
| Molecular weight: | 508 |
| Occurence in plants: |
Paxillus atrotomentosus [Fungi] »  ![Wikipedia: Paxillus atrotomentosus [Fungi]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
| |
| Canonical SMILES | CC(C1C(O1)C(C)(C2CCC3(C2(CC(C4C3=CC(=O)C5C4(CC(C(C5)O)O)C)O)C)O)O)C(C)(C)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2[C@@H](C[C@]2([C@]1(CC[C@@H]2[C@](C)([C@H]1[C@@H](C(C(C)(C)O)C)O1)O)O)C)O)C » 
C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2[C@@H](C[C@]2([C@]1(CC[C@@H]2[C@](C)([C@H]1[C@@H]([C@H](C(C)(C)O)C)O1)O)O)C)O)C » 
C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2[C@@H](C[C@]2([C@]1(CC[C@@H]2[C@](C)([C@H]1[C@@H]([C@@H](C(C)(C)O)C)O1)O)O)C)O)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,11R,13R,14S,17S)-2,3,11,14-tetrahydroxy-17-[(1R)-1-hydroxy-1-[(2R,3R)-3-(3-hydroxy-3-methylbutan-2-yl)oxiran-2-yl]ethyl]-10,13-dimethyl-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | | | PubChem CID | 101935917 | InChiKey
[ ChemIDPlus: search ] | XBKREFDHEBPCKQ-CUHMKKOTSA-N | | InChI | InChI=1S/C28H44O8/c1-13(24(2,3)33)22-23(36-22)27(6,34)20-7-8-28(35)15-10-16(29)14-9-17(30)18(31)11-25(14,4)21(15)19(32)12-26(20,28)5/h10,13-14,17-23,30-35H,7-9,11-12H2,1-6H3/t13?,14-,17+,18-,19+,20-,21+,22+,23+,25-,26+,27+,28+/m0/s1 |
| |
| EI-MS m/z (relative intensity %) | | | FAB-MS m/z | 531 (M+Na)+ (4), 509 (M+H)+ (10), 491 (14), 473 (3), 455 (2), 397 (3), 373 (3), 345 (4), 291 (22), 249 (14), 213 (11), 189 (11), 161 (19), 145 (19), 109 (30), 91 (44), 81 (50), 69 (44), 59 (100) | | HR-MS | |
|
|
| CD3OD | | 01 | 39.06 | | 02 | 68.93 | | 03 | 68.56 | | 04 | 33.30 | | 05 | 52.76 | | 06 | 206.66 | | 07 | 122.71 | | 08 | 165.67 | | 09 | 42.95 | | 10 | 39.89 | | 11 | 69.46 | | 12 | 43.52 | | 13 | 48.49 | | 14 | 84.72 | | 15 | 31.84 | | 16 | 21.88 | | 17 | 54.29 | | 18 | 18.78 | | 19 | 24.61 | | 20 | 72.77 | | 21 | 23.99 | | 22 | 66.97 | | 23 | 54.47 | | 24 | 47.56 | | 25 | 72.93 | | 26 | 28.03 | | 27 | 27.04 | | 28 | 12.42 |
|
| CD3OD | | 01-Ha | 1.37 (t, 12.8, 11.8) | | 01-He | 2.59 (dd, 12.8, 4.2) | | 02-Ha | 4.01 (dt, 11.8, 4.2, 3.6) | | 03-He | 3.96 (q ) | | 04-Ha | 1.78 (dt, 14.0, 13.2, 2.4) | | 04-He | 1.69 (dt, 14.0, 4.0, 3.4) | | 05-H | 2.34 (dd, 13.2, 4.0) | | 07-H | 5.81 (d, 2.5) | | 09-H | 3.16 (dd, 9.0, 2.5) | | 11-Ha | 4.10 (ddd, 10.5, 9.0, 6.0) | | 12-Ha | 2.25 (t, 12.2, 10.5) | | 12-He | 2.14 (dd, 12.2, 6.0) | | 15-Ha | 1.96 | | 15-Hb | 1.61 | | 16-Ha | 1.96 | | 16-Hb | 1.96 | | 17-H | 2.50 (dd, 9.5, 8.8) | | 18-Me | 0.844 (s ) | | 19-Me | 1.055 (s ) | | 21-Me | 1.319 (s ) | | 22-H | 2.92 (d, 2.3) | | 23-Ha | 2.88 (dd, 7.6, 2.3) | | 24-H | 1.24 (dq 7.6, 7.0, 7.0, 7.0) | | 26-Me | 1.233 (s ) | | 27-Me | 1.230 (s ) | | 28-Me | 1.019 (d, 7.0) |
|
| |
| M.P. | | | [α]D20 | | | IR (KBr) ν max (cm-1) | 3386 (OH), 1652 (cyclohexenone), 1589 (C=C) | | UV (EtOH) λ max (log ε) | |
| |
| NP-HPLC | column Silasorb 600 (4 x 250 mm), flow-rate 0.8 ml.min-1, solvent Et2O-hexane-MeOH-H2O (440:430:120:10), Ret 77.8 min (20E 47.5 min); solvent Et2O-CH3CN-H2O (780:190: 30), Ret 69.2 min (20E 31.7 min) | | RP-HPLC | column Separon SIX C18 (4 x 250 mm), solvent MeOH-H2O, linear gradient 10 to 70 % MeOH in 50 min, flow-rate 0.6 ml.min-1, Ret 41.9 min (20E 42.7 min). | | TLC | | | GLC | | | HPLC | |
| |
Drosophila melanogaster BII cell assay: EC50 = 5.0 x 10-5M |
| |
| |
Permanent link to this datasheet: 25-HYDROXYATROTOSTERONE B
|
|




![Wikipedia: Paxillus atrotomentosus [Fungi]](/images/wikipedia.png)

