|
|
|
|
Year of first isolation: |
1978 |
| Formula: | C28H44O7 |
| Molecular weight: | 492 |
| Occurence in plants: |
Rhaponticum integrifolium [Asteraceae] »  ![Wikipedia: Rhaponticum integrifolium [Asteraceae]](/images/wikipedia.png)
Leuzea carthamoides [Asteraceae] »  ![Wikipedia: Leuzea carthamoides [Asteraceae]](/images/wikipedia.png)
Diploclisia glaucescens [Menispermaceae] »  ![Wikipedia: Diploclisia glaucescens [Menispermaceae]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
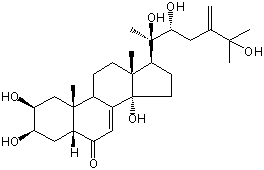
| |
| Canonical SMILES | CC12CCC3C(=CC(=O)C4C3(CC(C(C4)O)O)C)C1(CCC2C(C)(C(CC(=C)C(C)(C)O)O)O)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@](C)([C@@H](CC(=C)C(C)(C)O)O)O)O)C)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-17-[(2R,3R)-2,3,6-trihydroxy-6-methyl-5-methylideneheptan-2-yl]-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | | | PubChem CID | 13889301 | InChiKey
[ ChemIDPlus: search ] | XTSYLJLNVWBIFH-IJYGRGDZSA-N | | InChI | InChI=1S/C28H44O7/c1-15(24(2,3)33)11-23(32)27(6,34)22-8-10-28(35)17-12-19(29)18-13-20(30)21(31)14-25(18,4)16(17)7-9-26(22,28)5/h12,16,18,20-23,30-35H,1,7-11,13-14H2,2-6H3/t16-,18-,20+,21-,22-,23+,25+,26+,27+,28+/m0/s1 |
| |
| CI-MS (NH3) m/z | 510 (M+H+NH3)+, 493 (M+H)+, ... | | EI-MS m/z (relative intensity %) | 474 (M-18)+(3), 456 (11), 438 (15), 363 (66), 345 (100), 327 (47), 309 (13), 300 (26), 173 (17), 113 (37), 111 (55), 97 (52), 95 (63), 93 (35), 83 (91), 71 (62), 69 (62). | | HR-MS | |
|
|
| C5D5N | | 01 | 38.1 (t ) | | 02 | 68.1 (d ) | | 03 | 68.1 (d ) | | 04 | 32.5 (t ) | | 05 | 51.4 (d ) | | 06 | 203.3 (s ) | | 07 | 121.7 (d ) | | 08 | 166.0 (s ) | | 09 | 34.6 (d ) | | 10 | 38.7 (s ) | | 11 | 21.2 (t ) | | 12 | 31.9 (t ) | | 13 | 48.2 (s ) | | 14 | 84.2 (s ) | | 15 | 32.1 (t ) | | 16 | 21.5 (t ) | | 17 | 50.1 (d ) | | 18 | 17.9 (q ) | | 19 | 24.5 (q ) | | 20 | 76.8 (s ) | | 21 | 21.6 (q ) | | 22 | 78.0 (d ) | | 23 | 34.9 (t ) | | 24 | 156.2 (s ) | | 25 | 72.2 (s ) | | 26 | 30.2 (q )* | | 27 | 30.9 (q )* | | 28 | 109.6 (t ) |
| CD3OD | | 01 | 37.41 | | 02 | 68.72 | | 03 | 68.53 | | 04 | 32.84 | | 05 | 51.80 | | 06 | 206.42 | | 07 | 122.16 | | 08 | 167.88 | | 09 | 35.13 | | 10 | 39.27 | | 11 | 21.55 | | 12 | 32.51 | | 13 | 49.0* | | 14 | 85.28 | | 15 | 31.78 | | 16 | 21.55 | | 17 | 50.50 | | 18 | 18.04 | | 19 | 24.40 | | 20 | 78.04 | | 21 | 21.02 | | 22 | 77.81 | | 23 | 34.63 | | 24 | 155.36 | | 25 | 73.61 | | 26 | 29.76 | | 27 | 30.22 | | 28 | 110.38 |
|
| CD3OD | | 01-Ha | 1.43 (dd, 13.4; 12.0) | | 01-Hb | 1.80 (dd, 13.4; 4.2) | | 02-Ha | 3.84 (ddd, 12.0; 4.2; 3.0) | | 03-He | 3.95 (q, 3; 3; 3) | | 04-Ha | 1.73 | | 04-He | 1.70 | | 05-H | 2.39 (dd, 12.6; 4.4) | | 07-H | 5.82 (d, 2.6) | | 09-Ha | 3.16 (ddd, 11.5; 7.0; 2.6) | | 11-Ha | 1.70 | | 11-He | 1.82 | | 12-Ha | 2.14 | | 12-He | 1.89 | | 15-Ha | 1.98 | | 15-Hb | 1.61 | | 16-Ha | 2.04 | | 16-Hb | 1.80 | | 17-H | 2.42 (dd, 9.4; 8.0) | | 18-Me | 0.901 (s ) | | 19-Me | 0.971 (s ) | | 21-Me | 1.234 (s ) | | 22-Hb | 3.60 (d,d 10.7; 2.0) | | 23-Ha | 2.14 (dd, 14.4; 10.7) | | 23-Hb | 2.39 (dd, 14.4; 2.0) | | 26-Me | 1.321 (s ) | | 27-Me | 1.375 (s ) | | 28 =CH2 | 4.96 (b), 5.13 (b) |
| C5D5N | | 01-Ha | | | 01-Hb | | | 02-Ha | 3.75-4.20 (m ) | | 03-He | 3.75-4.20 (m ) | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | 6.08 | | 09-Ha | 3.45 | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 1.08 (s ) | | 19-Me | 0.95 (s ) | | 21-Me | 1.46 (s ) | | 22-Hb | | | 23-Ha | | | 23-Hb | | | 26-Me | 1.40 (s ) | | 27-Me | 1.46 (s ) | | 28 =CH2 | 5.14, 4.97 |
|
| |
| M.P. | 244-246 °C ; | | [α]D20 | +54.4 ? 2° (c 0.37; MeOH) | | IR (KBr) ν max (cm-1) | 3300-3500 (OH), 1665 | | UV (EtOH) λ max (log ε) | 245 (4.15) ; |
| |
| HPTLC | | | TLC | Rf 0.44 (EtOAc-MeOH-NH4OH 85:10:5); Rf 0.34 (CH2Cl2-EtOH 85:15). | | HPLC | NP-HPLC, (Zorbax-Sil) Ret 22.0 min (CH2Cl2-iPrOH-H2O, 125:25:2) (20E 53.2 min). RP-HPLC, (Spherisorb-5ODS2) Ret 8.6 min (solvent ACN-1? TFA in H2O 23:77 (v/v) (20E 5.5 min). |
| |
Drosophila melanogaster BII cell assay: EC50 = 4.0 x 10-9M | Galleria mellonella in vivo assay: ED50 = 12.5 ug/g | Sarcophaga bullata in vivo assay: ED50 = 10.4 ug/g |
| |
| First isolation | BALTAYEV, U.A. et al. (1978) Khim. Prir. Soedin. 463-465 |
 | | General | MILLER, R.W. et al. (1985) Planta Med. 46, 40-42 |
 | | General | GIRAULT, J.-P. et al. (1988) Phytochemistry 27, 737-741 |
 | | Bioactivities | SLÁMA, K. et al. (1993) Insect Biochem. Molec. Biol. 23, 181-185 |
 | | General | KUSAMBA, C. et al. (1995) Fitoterapia LXVI, 175-178 |
 | | Bioactivities | DINAN, L. et al. (1999) J. Computer-aided Mol. Design 13, 185-207 |
 | | Struct. analysis | BUDĚŠÍNSKÝ, M. et al. (2008) Steroids 73, 502-514 |
 |
| |
Permanent link to this datasheet: 24(28)-DEHYDROMAKISTERONE A
|
|




![Wikipedia: Rhaponticum integrifolium [Asteraceae]](/images/wikipedia.png)

