|
|
|
|
Year of first isolation: |
1973 |
| Formula: | C27H42O7 |
| Molecular weight: | 478 |
| Occurence in plants: |
Ipomoea calonyction [Convolvulaceae] »  ![Wikipedia: Ipomoea calonyction [Convolvulaceae]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
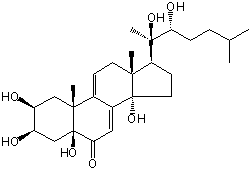
| |
| Canonical SMILES | CC(C)CCC(C(C)(C1CCC2(C1(CC=C3C2=CC(=O)C4(C3(CC(C(C4)O)O)C)O)C)O)O)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | CC(C)CC[C@H]([C@@](C)([C@H]1CC[C@@]2([C@@]1(CC=C3C2=CC(=O)[C@]4([C@@]3(C[C@@H]([C@@H](C4)O)O)C)O)C)O)O)O » 
| | IUPAC Name | (2S,3R,5S,10R,13R,14S,17S)-17-[(2R,3R)-2,3-dihydroxy-6-methylheptan-2-yl]-2,3,5,14-tetrahydroxy-10,13-dimethyl-1,2,3,4,12,15,16,17-octahydrocyclopenta[a]phenanthren-6-one | | CAS-RN | 52591-06-7 | | PubChem CID | 21723377 | InChiKey
[ ChemIDPlus: search ] | ZKUKSIKBNQQCOB-DPKIEXNISA-N | | InChI | InChI=1S/C27H42O7/c1-15(2)6-7-21(30)25(5,32)20-9-11-26(33)17-12-22(31)27(34)14-19(29)18(28)13-24(27,4)16(17)8-10-23(20,26)3/h8,12,15,18-21,28-30,32-34H,6-7,9-11,13-14H2,1-5H3/t18-,19+,20-,21+,23+,24+,25+,26+,27+/m0/s1 |
| |
| CI-MS (NH3) m/z | | | EI-MS m/z | 478 (M)+, 460, 445, 422, 377, 359 (base peak), 341, 323, 305, 145, 109, 105, 99, 91, 85, 83, 81 | | HR-MS | 478.2941 |
|
|
| C5D5N | | 01 | 38.6 | | 02 | 67.9 * | | 03 | 69.5 * | | 04 | 35.2 | | 05 | 79.7 | | 06 | 201.1 | | 07 | 116.8 | | 08 | 156.0 | | 09 | 137.4 | | 10 | 45.4 | | 11 | 132.9 | | 12 | 38.6 | | 13 | 47.4 | | 14 | 83.2 | | 15 | 31.3 | | 16 | 21.5 | | 17 | 49.9 | | 18 | 26.3 | | 19 | 17.9 | | 20 | 76.5 | | 21 | 21.2 | | 22 | 76.8 | | 23 | 30.2 | | 24 | 37.0 | | 25 | 28.2 | | 26 | 22.4 ** | | 27 | 23.3 ** |
|
| DMSO-d6 | | 01-Ha | | | 01-Hb | | | 02-Ha | | | 03-He | | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | 5.65 (s ) | | 09-Ha | | | 11-H | 6.18 (m, w1/2 = 10) | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 0.73 (s ) | | 19-Me | 0.96 (s ) | | 21-Me | 1.06 (s ) | | 22-H | | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 25-H | | | 26-Me | 0.86 (d, 7) | | 27-Me | 0.86 (d, 7) |
|
| |
| M.P. | 242-243 °C | | [α]D24 | + 79.3° (c ; MeOH) | | IR (KBr) ν max (cm-1) | 3600-3200 (OH), 1652 (cyclohexenone), 1604 | | UV (EtOH) λ max (log ε) | 298 (4.03) |
| |
| HPTLC | | | TLC | NP-TLC on silicagel Rf 0.49 (CHCl3-EtOH 4:1); RP-TLC on paraffin coated silica gel Rf 0.28 (MeOH-H2O 50:50); RP-TLC on C18-bonded silica gel Rf 0.26 (MeOH-H2O 65:35) | | GLC | GLC Ret 4.1 min on 1.5% OV101 (0.9 m x 4.5 mm i.d.) at 285°C after silylation at 140°C for 60 h. | | HPLC | |
| |
Drosophila melanogaster BII cell assay: EC50 = 3.4 x 10-7M |
| |
| First isolation | CANONICA, L. et al. (1973) Experientia 29, 1062-1063 |
 | | General | CANONICA, L. et al. (1975) Phytochemistry 14, 525-527 |
 | | General | CANONICA, L. et al. (1977) Gazz. Chim. Ital. 107, 123-130 |
 | | General | KREPINSKY, J. et al. (1977) Org. Magn. Reson. 10, 255-257 |
 | | General | WILSON, I.D. et al. (1985) J. Chromatogr. 318, 373-377 |
 | | General | BIELBY, C.R. et al. (1986) J. Chromatogr. 351, 57-64 |
 | | Bioactivities | BOURNE, P. et al. (2002) J. Insect Sci. 2:11 (pp. 11) |
 |
| |
Permanent link to this datasheet: KALADASTERONE
|
|




![Wikipedia: Ipomoea calonyction [Convolvulaceae]](/images/wikipedia.png)

