|
|
|
|
Year of first isolation: |
1975 |
| Formula: | C27H44O8 |
| Molecular weight: | 496 |
| Occurence in plants: |
Ajuga turkestanica [Lamiaceae (alt. Labiatae)] »  ![Wikipedia: Ajuga turkestanica [Lamiaceae (alt. Labiatae)]](/images/wikipedia.png)
Vitex glabrata [Lamiaceae (alt. Labiatae)] »  ![Wikipedia: Vitex glabrata [Lamiaceae (alt. Labiatae)]](/images/wikipedia.png)
Vitex canescens [Lamiaceae (alt. Labiatae)] »  ![Wikipedia: Vitex canescens [Lamiaceae (alt. Labiatae)]](/images/wikipedia.png)
Tapinella panuoides [Fungi] »  ![Wikipedia: Tapinella panuoides [Fungi]](/images/wikipedia.png)
Leuzea carthamoides [Asteraceae] »  ![Wikipedia: Leuzea carthamoides [Asteraceae]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
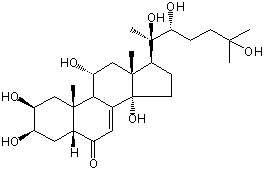
| |
| Canonical SMILES | CC12CC(C3C(=CC(=O)C4C3(CC(C(C4)O)O)C)C1(CCC2C(C)(C(CCC(C)(C)O)O)O)O)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2[C@@H](C[C@]2([C@]1(CC[C@@H]2[C@](C)([C@@H](CCC(C)(C)O)O)O)O)C)O)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,11R,13R,14S,17S)-2,3,11,14-tetrahydroxy-10,13-dimethyl-17-[(2R,3R)-2,3,6-trihydroxy-6-methylheptan-2-yl]-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | 41451-87-0 | | PubChem CID | 14376672 | InChiKey
[ ChemIDPlus: search ] | WSBAGDDNVWTLOM-XHZKDPLLSA-N | | InChI | InChI=1S/C27H44O8/c1-23(2,33)8-7-21(32)26(5,34)20-6-9-27(35)15-11-16(28)14-10-17(29)18(30)12-24(14,3)22(15)19(31)13-25(20,27)4/h11,14,17-22,29-35H,6-10,12-13H2,1-5H3/t14-,17+,18-,19+,20-,21+,22+,24-,25+,26+,27+/m0/s1 |
| |
| CI-MS (NH3) m/z | | | EI-MS m/z (relative intensity %) | 460 [M-2H2O], 442, 424, 409, 379, 361, 343, 325, 300, 143, 125, 99, 81, 69. | | HR-MS | |
|
|
| C5D5N | | 01 | 39.6 | | 02 | 68.0 * | | 03 | 68.2 * | | 04 | 32.6 | | 05 | 52.5 | | 06 | 204.0 | | 07 | 122.1 | | 08 | 164.2 | | 09 | 42.4 | | 10 | 39.3 | | 11 | 68.7 * | | 12 | 43.9 | | 13 | 48.0 | | 14 | 84.1 | | 15 | 31.7 | | 16 | 21.4 | | 17 | 49.8 | | 18 | 18.7 | | 19 | 24.6 | | 20 | 76.7 | | 21 | 21.4 | | 22 | 77.4 | | 23 | 27.2 | | 24 | 42.5 | | 25 | 69.5 | | 26 | 29.8 | | 27 | 29.8 |
| CD3OD | | 01 | 39.09 | | 02 | 68.94 | | 03 | 68.57 | | 04 | 33.28 | | 05 | 52.78 | | 06 | 206.66 | | 07 | 122.74 | | 08 | 165.74 | | 09 | 42.94 | | 10 | 39.91 | | 11 | 69.51 | | 12 | 43.79 | | 13 | a | | 14 | 84.87 | | 15 | 31.86 | | 16 | 21.52 | | 17 | 50.35 | | 18 | 18.89 | | 19 | 24.62 | | 20 | 77.83 | | 21 | 21.02 | | 22 | 78.42 | | 23 | 27.35 | | 24 | 42.40 | | 25 | 71.29 | | 26 | 29.73 | | 27 | 28.95 |
|
| C5D5N | | 01-Ha | | | 01-He | | | 02-Ha | 4.45 | | 03-He | 4.06 | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | 6.12 | | 09-Ha | 3.75 | | 11-Ha | 4.45 | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 1.12 (s ) | | 19-Me | 1.18 (s ) | | 21-Me | 1.45 (s ) | | 22-Hb | 3.75 | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.24 (s ) | | 27-Me | 1.24 (s ) |
| CD3OD | | 01-Ha | 1.38 (dd, 13.0, 12.0) | | 01-He | 2.59 dd, 13.0, 4.3) | | 02-Ha | 4.01 (ddd, 12.0, 4.3, 3.4) | | 03-He | 3.95 (q ) | | 04-Ha | 1.78 | | 04-He | 1.68 (dt, 14.2, 3.9, 3.5) | | 05-H | 2.33 (dd, 13.2, 3.9) | | 07-H | 5.80 (dd, 2.6, 1.0) | | 09-Ha | 3.15 (dd, 9.0, 2.6) | | 11-Ha | 4.10 (ddd, 10.6, 9.0, 6.2) | | 12-Ha | 2.22 (dd, 12.4, 10.6) | | 12-He | 2.16 (dd, 12.4, 6.2) | | 15-Ha | 1.95 | | 15-Hb | 1.58 | | 16-Ha | 2.00 | | 16-Hb | 1.75 | | 17-H | 2.43 | | 18-Me | 0.877 (s ) | | 19-Me | 1.057 (s ) | | 21-Me | 1.193 (s ) | | 22-Hb | 3.32 (dd, 11.0, 1.8) | | 23-Ha | 1.66 | | 23-Hb | 1.29 | | 24-Ha | 1.80 | | 24-Hb | 1.43 (ddd, 13.6, 11.6, 4.3) | | 26-Me | 1.206 (s ) | | 27-Me | 1.220 (s ) |
|
| |
| M.P. | | | [α]D20 | | | IR (KBr) ν max (cm-1) | 3300-3500 (OH), 1660 (CO) | | UV (EtOH) λ max (log ε) | 244 (3.95) |
| |
| NP-HPLC | column Silasorb 600 (4 x 250 mm), flow-rate 0.8 ml.min-1, solvent Et2O-hexane-MeOH-H2O (440:430:120:10), Ret 97.9 min (20E 47.5 min) | | HPTLC | | | TLC | | | GLC | | | RP-HPLC | column Separon SIX C18 (4 x 250 mm), solvent MeOH-H2O, linear gradient 10 to 70 % MeOH in 50 min, flow-rate 0.6 ml.min-1, Ret 33.9 min (20E 42.7 min). |
| |
Drosophila melanogaster BII cell assay: EC50 = 1.3 x 10-6M | Drosophila melanogaster BII cell assay: EC50 = 3.0 x 10-7M | Drosophila melanogaster BII cell assay: EC50 = 8.0 x 10-7M | Galleria mellonella in vivo assay: ED50 = 62.5 ug/g | Sarcophaga bullata in vivo assay: ED50 = 0.3 ug/g | Dermestes vulpinus in vivo assay: ED50 = 4.2 ug/g |
| |
| First isolation | USMANOV, B.Z. et al. (1975) Khim. Prir. Soedin. 466-470 |
 | | General | WERAWATTANAMETIN, K. et al. (1986) J. Nat. Prod. 49, 365-366 |
 | | Bioactivities | CLEMENT, C.Y. et al. (1993) Insect Biochem. Molec. Biol. 23, 187-193 |
 | | Bioactivities | SLÁMA, K. et al. (1993) Insect Biochem. Molec. Biol. 23, 181-185 |
 | | General | SUKSAMRARN, A. et al. (1997) Phytochemistry 45, 1149-1152 |
 | | Identification | VOKÁČ, K. et al. (1998) Phytochemistry 49, 2109-2114 |
 | | Bioactivities | DINAN, L. et al. (1999) J. Computer-aided Mol. Design 13, 185-207 |
 | | Bioactivities | DINAN, L. et al. (2005) In: Comprehensive Molecular Insect Science (eds. L. I. Gilbert, C. Iatrou, S. Gill), Elsevier, Oxford, III, 197-242 |
 | | General | BUDĚŠÍNSKÝ, M. et al. (2008) Steroids 73, 502-514 |
 |
| |
Permanent link to this datasheet: TURKESTERONE
|
|




![Wikipedia: Ajuga turkestanica [Lamiaceae (alt. Labiatae)]](/images/wikipedia.png)

