|
|
|
|
Year of first isolation: |
1999 |
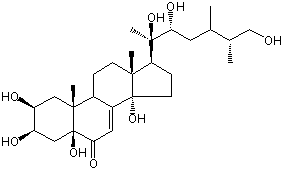
| Formula: | C28H46O8 |
| Molecular weight: | 510 |
| Occurence in plants: |
|
|
| Occurence in animals: |
Palythoa australiae [Zoanthidae] »  ![Wikipedia: Palythoa australiae [Zoanthidae]](/images/wikipedia.png)
|
|
| |
| Canonical SMILES | CC(CC(C(C)(C1CCC2(C1(CCC3C2=CC(=O)C4(C3(CC(C(C4)O)O)C)O)C)O)O)O)C(C)CO | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@](C[C@H]([C@H]1O)O)(C(=O)C=C1C2CC[C@]2([C@]1(CC[C@@H]2[C@]([C@@H](CC([C@H](CO)C)C)O)(O)C)O)C)O)C » 
C1[C@]2([C@@](C[C@H]([C@H]1O)O)(C(=O)C=C1C2CC[C@]2([C@]1(CC[C@@H]2[C@]([C@@H](C[C@H]([C@H](CO)C)C)O)(O)C)O)C)O)C » 
C1[C@]2([C@@](C[C@H]([C@H]1O)O)(C(=O)C=C1C2CC[C@]2([C@]1(CC[C@@H]2[C@]([C@@H](C[C@@H]([C@H](CO)C)C)O)(O)C)O)C)O)C » 
| | IUPAC Name | (2S,3R,5S,9R,10R,13R,14S,17S)-2,3,5,14-tetrahydroxy-10,13-dimethyl-17-[(2R,3R,5S,6R)-2,3,7-trihydroxy-5,6-dimethylheptan-2-yl]-1,2,3,4,9,11,12,15,16,17-decahydrocyclopenta[a]phenanthren-6-one | | CAS-RN | | | PubChem CID | 100956102 | InChiKey
[ ChemIDPlus: search ] | SCHNJHTVEMNUAG-AHAWHJSRSA-N | | InChI | InChI=1S/C28H46O8/c1-15(16(2)14-29)10-22(32)26(5,34)21-7-9-27(35)18-11-23(33)28(36)13-20(31)19(30)12-25(28,4)17(18)6-8-24(21,27)3/h11,15-17,19-22,29-32,34-36H,6-10,12-14H2,1-5H3/t15-,16-,17-,19-,20+,21-,22+,24+,25+,26+,27+,28+/m0/s1 |
| |
| CI-MS (NH3) m/z | | | EI-MS m/z (relative intensity %) | | | HR-FAB-MS | 511.3263 [M+H]+, - 0.8 mmu |
|
|
| C5D5N | | 01 | 35.0 (t) | | 02 | 68.0 (d) | | 03 | 69.9 (d) | | 04 | 36.1 (t) | | 05 | 80.0 (s) | | 06 | 200.9 (s) | | 07 | 119.8 (d) | | 08 | 166.9 (s) | | 09 | 38.4 (d) | | 10 | 44.9 (s) | | 11 | 21.5 (t) | | 12 | 31.7 (t) | | 13 | 48.2 (s) | | 14 | 84.0 (s) | | 15 | 32.2 (t) | | 16 | 22.2 (t) | | 17 | 49.9 (d) | | 18 | 18.0 (q) | | 19 | 17.3 (q) | | 20 | 76.8 (s) | | 21 | 21.6 (q) | | 22 | 74.1 (d) | | 23 | 37.9 (t) | | 24 | 31.0 (d) | | 25 | 37.7 (d) | | 26 | 66.8 (t) | | 27 | 10.9 (q) | | 28 | 15.9 (q) |
|
| C5D5N | | 01-Ha | 2.21 (s) | | 01-He | 2.10 (m) | | 02-Ha | 4.25 (br d 11.8) | | 03-He | 4.15 (br, s) | | 04-Ha | 2.13 (m) | | 04-He | 1.99 (dd) | | 07-H | 6.28 (d) | | 09-H | 3.65 (m) | | 11-Ha | 1.91 (m) | | 11-He | 1.83 (m) | | 12-Ha | 2.18 (m) | | 12-He | 1.81 (m) | | 15-Ha | 2.60 (dt) | | 15-Hb | 2.03 (m) | | 16-Ha | 2.44 (m) | | 16-Hb | 2.05 (m) | | 17-H | 2.93 (t) | | 18-Me | 1.22 (s) | | 19-Me | 1.16 (s) | | 21-Me | 1.58 (s) | | 22-H | 3.99 (d) | | 23-Ha | 1.88 (d) | | 23-Hb | 1.54 (m) | | 24-H | 2.54 (m) | | 25-H | 2.24 (m) | | 26-Ha | 3.79 (dd) | | 26-Hb | 3.72 (dd) | | 27-Me | 0.92 (d) | | 28-Me | 0.91 (d) |
|
| |
| M.P. | | | [α]D26 | + 87 ? ° (c 2.2; MeOH) | | IR (film) ν max (cm-1) | 3435, 1635 | | UV (MeOH) λ max (log ε) | 243 (4.0) |
| |
| |
| |
| |
Permanent link to this datasheet: PALYTHOALONE A
|
|




![Wikipedia: Palythoa australiae [Zoanthidae]](/images/wikipedia.png)

