|
|
|
|
Year of first isolation: |
1966 |
| Formula: | C27H44O7 |
| Molecular weight: | 480 |
| Occurence in plants: |
many plant species including Fungi [Plantae] »  ![Wikipedia: many plant species including Fungi [Plantae]](/images/wikipedia.png)
many seed plants, bryophytes, ferns [Plantae] »  ![Wikipedia: many seed plants, bryophytes, ferns [Plantae]](/images/wikipedia.png)
|
| Occurence in animals: |
Bombyx mori [Bombycidae] »  ![Wikipedia: Bombyx mori [Bombycidae]](/images/wikipedia.png)
Jasus lalandii [Crustaceae] »  ![Wikipedia: Jasus lalandii [Crustaceae]](/images/wikipedia.png)
many Arthropods and Invertebrates »  
Molluscs [Mollusca] »  ![Wikipedia: Molluscs [Mollusca]](/images/wikipedia.png)
Helminths [Helminths] »  ![Wikipedia: Helminths [Helminths]](/images/wikipedia.png)
|
|
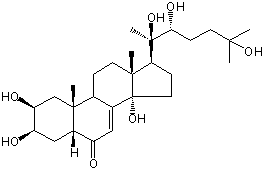
| |
| Canonical SMILES | CC12CCC3C(=CC(=O)C4C3(CC(C(C4)O)O)C)C1(CCC2C(C)(C(CCC(C)(C)O)O)O)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@@](C)(O)[C@@H](CCC(C)(C)O)O)O)C)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-17-[(2R,3R)-2,3,6-trihydroxy-6-methylheptan-2-yl]-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | 5289-74-7 | | PubChem CID | 5459840 | InChiKey
[ ChemIDPlus: search ] | NKDFYOWSKOHCCO-YPVLXUMRSA-N | | InChI | nChI=1S/C27H44O7/c1-23(2,32)9-8-22(31)26(5,33)21-7-11-27(34)16-12-18(28)17-13-19(29)20(30)14-24(17,3)15(16)6-10-25(21,27)4/h12,15,17,19-22,29-34H,6-11,13-14H2,1-5H3/t15-,17-,19+,20-,21-,22+,24+,25+,26+,27+/m0/s1 |
| |
| CI-MS (NH3) m/z | 498 (M+H+NH3)+, 481 (MH)+, 463, 445, 427, 363, 345, 329, 327, 320, 160 | | EI-MS m/z (relative intensity %) | 480 (<1), 462 (1), 444 (1), 429 (3), 426 (12), 411 (2), 408 (3), 393 (1), 363 (7), 346 (11), 345 (30), 344 (26), 328 (17), 327 (19), 300 (13), 145 (8), 143 (8), 99 (100), 81 (27) | | FAB-MS m/z | 479 (M-H)- |
|
|
| C5D5N | | 01 | 38.09 (t ) | | 02 | 68.33 (d ) | | 03 | 68.23 (d ) | | 04 | 32.53 (t ) | | 05 | 51.48 (d ) | | 06 | 203.56 (s ) | | 07 | 121.79 (d ) | | 08 | 166.11 (s ) | | 09 | 34.67 (d ) | | 10 | 38.80 (s ) | | 11 | 21.29 (t ) | | 12 | 32.19 (t ) | | 13 | 48.27 (s ) | | 14 | 84.42 (s ) | | 15 | 31.88 (t ) | | 16 | 21.61 (t ) | | 17 | 50.28 (d ) | | 18 | 17.99 (q ) | | 19 | 24.55 (q ) | | 20 | 77.09 (s ) | | 21 | 21.77 (q ) | | 22 | 77.75 (d ) | | 23 | 27.59 (t ) | | 24 | 42.64 (t ) | | 25 | 69.86 (s ) | | 26 | 30.10 (q ) | | 27 | 30.15 (q ) |
| CD3OD | | 01 | 37.33 | | 02 | 68.68 | | 03 | 68.50 | | 04 | 32.86 | | 05 | 51.78 | | 06 | 206.46 | | 07 | 122.13 | | 08 | 167.98 | | 09 | 35.07 | | 10 | 39.26 | | 11 | 21.48 | | 12 | 32.50 | | 13 | 48.60 | | 14 | 85.21 | | 15 | 31.78 | | 16 | 21.48 | | 17 | 50.51 | | 18 | 18.06 | | 19 | 24.41 | | 20 | 77.90 | | 21 | 21.04 | | 22 | 78.41 | | 23 | 23.32 | | 24 | 42.39 | | 25 | 71.30 | | 26 | 28.92 | | 27 | 29.72 |
|
| C5D5N | | 01-Ha | 1.91 | | 01-He | 2.14 | | 02-Ha | 4.17 (m, w1/2=22) | | 03-He | 4.21 (m, w1/2=8) | | 04-Ha | 1.80 | | 04-He | 2.01 | | 05-H | 3.01 | | 07-H | 6.25 (d, 2.5) | | 09-Ha | 3.58 (m, w1/2=22) | | 11-Ha | 1.71 | | 11-He | 1.88 | | 12-Ha | 2.58 (ddd, 13,13,5) | | 12-He | 1.95 | | 15-Ha | 2.14* | | 15-Hb | 1.89* | | 16-Ha | 2.44$ | | 16-Hb | 2.08$ | | 17-H | 3.00 | | 18-Me | 1.21 (s ) | | 19-Me | 1.06 (s ) | | 21-Me | 1.58 (s ) | | 22-H | 3.87 (m, w1/2=16) | | 23-Ha | 1.85 | | 23-Hb | 2.14 | | 24-Ha | 2.28 | | 24-Hb | 1.81 | | 26-Me | 1.36 (s ) | | 27-Me | 1.36 (s ) |
| CD3OD | | 01-Ha | 1.43 | | 01-He | 1.78 | | 02-Ha | 3.83 (m, w1/2=22) | | 03-He | 3.94 (m, w1/2=8) | | 04-Ha | 1.65 | | 04-He | 1.75 | | 05-H | 2.38 (dd, 13, 4) | | 07-H | 5.85 (d, 2.5) | | 09-Ha | 3.09 (m, w1/2=21) | | 11-Ha | 1.65 | | 11-He | 1.78 | | 12-Ha | 2.13 (ddd, 13,13,5) | | 12-He | 1.85 | | 15-Ha | 2.00 | | 15-Hb | 1.55 | | 16-Ha | 1.95 | | 16-Hb | 1.75 | | 17-H | 2.39 (m ) | | 18-Me | 0.89 (s ) | | 19-Me | 0.96 (s ) | | 21-Me | 1.18 (s ) | | 22-H | 3.33 (dd, 11, 2) | | 23-Ha | 1.30 | | 23-Hb | 1.65 | | 24-Ha | 1.75 | | 24-Hb | 1.45 | | 26-Me | 1.19 (s ) | | 27-Me | 1.20 (s ) |
|
| |
| M.P. | 241-242.5 °C ; | | [α]D20 | + 61.8 ° (c ; MeOH) | | IR (KBr) ν max (cm-1) | 3500 (OH), 1645 (cyclohexenone); | | UV (EtOH) λ max (log ε) | 240 (4.103) ; |
| |
| TLC | NP-TLC on silica gel Rf 0.15 (CHCl3-EtOH 4:1); RP-TLC on paraffin coated silica gel Rf 0.49 (MeOH-H2O 1:1); RP-TLC on C18-bonded silica gel Rf 0.47 (MeOH-H2O 65:35) | | GLC | Ret 7.55 min on 1.5% OV101 (0.9 m x 4 mm i.d.) at 285°C after silylation at 140°C for 60 h (presumed 2,3,14,20,22,25-hexakis-TMS ether). | | HPLC | 1- Retention time 16.8 min [Zorbax-Sil 250 mm x 4.6 mm i.d., solvent CH2Cl2-iPrOH-H2O 125:40:3 v/v/v, flow-rate 1 ml.min-1]; 2-Retention time 5.5 min [Spherisorb-5ODS2 250 mm x 4.6 mm i.d., solvent ACN-1? TFA in -H2O, 23:77 v/v, flow-rate 1 ml.min-1] |
| |
Drosophila melanogaster BII cell assay: EC50 = 7.6 x 10-9M | Drosophila melanogaster BII cell assay: EC50 = 7.5 x 10-9M | Calliphora vicina imaginal disc assay: EC50 = 7.9 x 10-8M | Drosophila melanogaster imaginal disc assay: EC50 = 4.2 x 10-8M | Drosophila melanogaster imaginal disc assay: EC50 = 6.0 x 10-8M | Drosophila melanogaster Kc-H cell assay: EC50 = 1.4 x 10-8M | Galleria mellonella in vivo assay: ED50 = 7.8 ug/g | Dermestes vulpinus in vivo assay: ED50 = 42.0 ug/g | Sarcophaga bullata in vivo assay: ED50 = 2.6 ug/g | Calliphora bioassay: 100% (20-hydroxyecdysone = 100%) | Musca bioassay: 100% (20-hydroxyecdysone = 100%) |
| |
| First isolation | HOCKS, P. et al. (1966) Tetrahedron Lett. 2989-2993 |
 | | First isolation | HOFFMEISTER, H. et al. (1966) Tetrahedron Lett. 4017-4023 |
 | | First isolation | HAMPSHIRE, F. et al. (1966) J. Chem. Soc., Chem. Commun. 37-38 |
 | | First isolation | CHONG, Y.K. et al. (1970) J. Chem. Soc., Chem. Commun. 1217-1218 |
 | | Bioactivities | CHIHARA, C.J. et al. (1972) J. Insect Physiol. 18, 1115-1123 |
 | | Bioactivities | YUND, M.A. et al. (1980) In: Invert. Systems in Vitro pp. 229-237 |
 | | Bioactivities | CHERBAS, L. et al. (1980) W. Roux Arch. Devel. Biol. 189, 1-15 |
 | | Bioactivities | BERGAMASCO, R. et al. (1980) In: Progress in Ecdysone Research (Ed. J.A. Hoffmann), Elsevier/North Holland Biomed. Press, Amsterdam 299-324 |
 | | General | KUBO, I. et al. (1985) Agric. Biol. Chem. 49, 243-244 |
 | | General | WILSON, I.D. et al. (1985) J. Chromatogr. 318, 373-377 |
 | | General | BIELBY, C.R. et al. (1986) J. Chromatogr. 351, 57-64 |
 | | General | EVERSHED, R.P. et al. (1987) J. Chromatogr. 390, 357-369 |
 | | General | GIRAULT, J.-P. et al. (1988) J. Insect Physiol. 34, 701-706 |
 | | Bioactivities | TERENTIOU, P. et al. (1993) Insect Biochem. Molec. Biol. 23, 131-136 |
 | | Bioactivities | SLÁMA, K. et al. (1993) Insect Biochem. Molec. Biol. 23, 181-185 |
 | | Bioactivities | CLEMENT, C.Y. et al. (1993) Insect Biochem. Molec. Biol. 23, 187-193 |
 | | Identification | COLL, J. et al. (1994) Tetrahedron 50, 7247-7252 |
 | | Bioactivities | DINAN, L. et al. (1999) J. Computer-aided Mol. Design 13, 185-207 |
 | | Bioactivities | SUKSAMRARN, A. et al. (2002) Insect Biochem. Molec. Biol. 32, 193-197 |
 | | Identification | BUDĚŠÍNSKÝ, M. et al. (2008) Steroids 73, 502-514 |
 |
| |
Permanent link to this datasheet: 20-HYDROXYECDYSONE
|
|




![Wikipedia: many plant species including Fungi [Plantae]](/images/wikipedia.png)

