|
|
|
|
Year of first isolation: |
2015 |
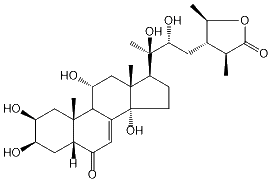
| Formula: | C29H44O9 |
| Molecular weight: | 536 |
| Occurence in plants: |
Ajuga turkestanica [Lamiaceae (alt. Labiatae)] »  ![Wikipedia: Ajuga turkestanica [Lamiaceae (alt. Labiatae)]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
| |
| Canonical SMILES | | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2[C@@H](C[C@]2([C@]1(CC[C@@H]2[C@](C)([C@@H](C[C@@H]1[C@H](OC(=O)[C@H]1C)C)O)O)O)C)O)C » 
| | IUPAC Name | (3S,4S,5R)-4-((2R,3R)-2,3-dihydroxy-3-((2S,3R,5R,9R,10R,11R,13R,14S,17S)-2,3,11,14-tetrahydroxy-10,13-dimethyl-6-oxo-2,3,4,5,6,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yl)butyl)-3,5-dimethyldihydrofuran-2(3H)-one | | CAS-RN | | | PubChem CID | | InChiKey
[ ChemIDPlus: search ] | GYHHEOJCLAGQCK-HNWGTDKPSA-N | | InChI | InChI=1S/C29H44O9/c1-13-15(14(2)38-25(13)35)8-23(34)28(5,36)22-6-7-29(37)17-10-18(30)16-9-19(31)20(32)11-26(16,3)24(17)21(33)12-27(22,29)4/h10,13-16,19-24,31-34,36-37H,6-9,11-12H2,1-5H3/t13-,14+,15-,16-,19+,20-,21+,22-,23+,24+,26-,27+,28+,29+/m0/s1 |
| |
| ESI-MS m/z | 537 [M+H]+, 519 [MH - H2O]+, 501 [MH - 2H2O]+, 483 [MH - 3H2O]+, 465 [MH - 3H2O]+ | | HRESI-MS | 559.28775 [M+Na]+, expected 559.28775 for C29H44O9Na | | EI-MS m/z (relative intensity %) | |
|
|
| D2O | | 01 | 39.2 | | 02 | 69.3 | | 03 | 69.3 | | 04 | 33.6 | | 05 | 53.4 | | 06 | 210.6 | | 07 | 123.9 | | 08 | 167.9 | | 09 | 43.4 | | 10 | 40.3 | | 11 | 70.3 | | 12 | 43.8 | | 13 | 49.3 | | 14 | 86.6 | | 15 | 32.5 | | 16 | 22.1 | | 17 | 50.7 | | 18 | 19.7 | | 19 | 25.3 | | 20 | 79.9 | | 21 | 21.3 | | 22 | 75.9 | | 23 | 35.2 | | 24 | 49.7 | | 25 | 44.4 | | 26 | 185.9 | | 27 | 17.0 | | 28 | 84.1 | | 29 | 20.4 |
|
| D2O | | 01-Ha | 1.39 (t, 13) | | 01-He | 2.48 (dd, 13.2, 4) | | 02-Ha | 4.09 (m, w1/2 = 11) | | 03-He | 4.09 (m, w1/2 = 11) | | 04-Ha | 1.77 | | 04-He | 1.77 | | 05-H | 2.31 | | 07-H | 5.99 (d, 2.6) | | 09-H | 3.13 (dd, 8.8, 2.6) | | 11-Ha | 4.23 (m, w1/2 = 27, ddd 10.8, 9, 6.1) | | 12-Ha | 2.07 | | 12-He | 2.28 (dd, 12.7, 6.1) | | 15-Ha | 2.06 | | 15-Hb | 1.66 (m) | | 16-Ha | 1.93 | | 16-Hb | 1.83 | | 17-H | 2.32 (m) | | 18-Me | 0.87 (s) | | 19-Me | 1.10 (s) | | 21-Me | 1.26 (s) | | 22-H | 3.63 (d, 10.8) | | 23-Ha | 1.59 (m) | | 23-Hb | 1.76 | | 24-Ha | 2.08 | | 25-H | 2.67 (dq, 10.8, 7.1) | | 27-Me | 1.32 (d, 7.2) | | 28-H | 4.34 (dq, 9.4, 6.1) | | 29-Me | 1.45 (d, 6.1) |
|
| |
| M.P. | °C ; | | [α]D20 | ° (c ; MeOH) | | IR (KBr) ν max (cm-1) | | | UV (EtOH) λ max (log ε) | () ; |
| |
| |
| |
| |
Permanent link to this datasheet: 11-HYDROXY-CYASTERONE
|
|




![Wikipedia: Ajuga turkestanica [Lamiaceae (alt. Labiatae)]](/images/wikipedia.png)

