|
|
|
|
Year of first isolation: |
1986 |
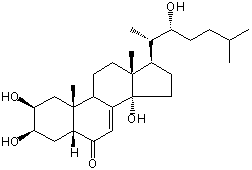
| Formula: | C27H44O5 |
| Molecular weight: | 448 |
| Occurence in plants: |
|
|
| Occurence in animals: |
Carcinus maenas [Crustaceae] »  ![Wikipedia: Carcinus maenas [Crustaceae]](/images/wikipedia.png)
|
|
| |
| Canonical SMILES | CC(C)CCC(C(C)C1CCC2(C1(CCC3C2=CC(=O)C4C3(CC(C(C4)O)O)C)C)O)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@H](C)[C@@H](CCC(C)C)O)O)C)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,13R,14S,17R)-2,3,14-trihydroxy-17-[(2S,3R)-3-hydroxy-6-methylheptan-2-yl]-10,13-dimethyl-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | 22005-50-1 | | PubChem CID | 10994017 | InChiKey
[ ChemIDPlus: search ] | HHQGPNBMKUXKRM-JUJQDXCZSA-N | | InChI | InChI=1S/C27H44O5/c1-15(2)6-7-21(28)16(3)17-9-11-27(32)19-12-22(29)20-13-23(30)24(31)14-25(20,4)18(19)8-10-26(17,27)5/h12,15-18,20-21,23-24,28,30-32H,6-11,13-14H2,1-5H3/t16-,17+,18-,20-,21+,23+,24-,25+,26+,27+/m0/s1 |
| |
| CI-MS (NH3) m/z | 466 (M+H+NH3)+, 449 (M+H)+, 431, 349, 331 | | EI-MS m/z (relative intensity %) | 448 (M)+, 430 (M-18) | | HR-MS | |
|
|
| C5D5N | | 01 | | | 02 | | | 03 | | | 04 | | | 05 | | | 06 | | | 07 | | | 08 | | | 09 | | | 10 | | | 11 | | | 12 | | | 13 | | | 14 | | | 15 | | | 16 | | | 17 | | | 18 | | | 19 | | | 20 | | | 21 | | | 22 | | | 23 | | | 24 | | | 25 | | | 26 | | | 27 | | | 28 | | | 29 | |
|
| C5D5N | | 01-Ha | | | 01-He | | | 02-Ha | 3.90 (m, w1/2 = 8) | | 03-He | 4.03 (m, w1/2 = 22) | | 04-Ha | | | 04-He | | | 05-H | 2.42 (dd, 13.5, 4) | | 07-H | 5.84 (d, 2.5) | | 09-Ha | 2.98 (m, w1/2 = 22) | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 14-H | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 0.68 (s ) | | 19-Me | 0.97 (s ) | | 21-Me | 0.92 (d, 6.5) | | 22-Hb | 3.62 (m, w1/2 = 13) | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 25-H | | | 26-Me | 0.88 (d, 6.5) | | 27-Me | 0.89 (d, 6.5) |
| CDCl3 | | 01-Ha | | | 01-He | | | 02-Ha | | | 03-He | | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | 6.16 (bs ) | | 09-Ha | | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 14-H | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 0.72 (s ) | | 19-Me | 1.05 (s ) | | 21-Me | 1.24 (d, 6.5) | | 22-Hb | | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 25-H | | | 26-Me | 0.83 (d, 6) | | 27-Me | 0.83 (d, 6) |
|
| |
| M.P. | | | [α]D20 | | | IR (KBr) ν max (cm-1) | | | UV (EtOH) λ max (log ε) | 241 (4,117) ; |
| |
| HPTLC | | | TLC | | | GLC | | | HPLC | NP-HPLC, Retention time -- min [ Zorbax-Sil 250 mm x 4.6 mm i.d., solvent CH2Cl2-iPrOH-H2O 125:15:1 v/v/v, flow-rate 1 ml.min-1]; RP-HPLC, Retention time 15.6 min [Zorbax-ODS 250 mm x 4.6 mm i.d., solvent ACN-1? TFA in H2O 35:65 v/v, flow-rate 1 ml.min-1] |
| |
Drosophila melanogaster BII cell assay: EC50 = 1.0 x 10-8M | Calliphora assay: 7% (20-hydroxyecdysone = 100%) |
| |
| General | HAFFERL, W. et al. (1972) J. Labelled Compd. 8, 81-94 |
 | | Bioactivities | BERGAMASCO, R. et al. (1980) In: Progress in Ecdysone Research (Ed. J.A. Hoffmann), Elsevier/North Holland Biomed. Press, Amsterdam 299-324 |
 | | First isolation | LACHAISE, F. et al. (1986) Mol. Cell. Endocrinol. 43, 253-261 |
 | | General | LACHAISE, F. et al. (1988) J. Insect Physiol. 34, 557-562 |
 | | General | LACHAISE, F. et al. (1989) J. Exp. Zool. 252, 283-292 |
 | | General | PIS, J. et al. (1995) Steroids 60, 188-194 |
 | | Bioactivities | DINAN, L. et al. (1999) J. Computer-aided Mol. Design 13, 185-207 |
 |
| |
Permanent link to this datasheet: 25-DEOXYECDYSONE
|
|




![Wikipedia: Carcinus maenas [Crustaceae]](/images/wikipedia.png)

