|
|
|
|
Year of first isolation: |
1972 |
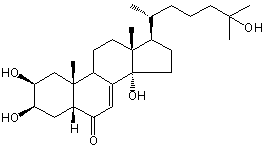
| Formula: | C27H44O5 |
| Molecular weight: | 448 |
| Occurence in plants: |
|
|
| Occurence in animals: |
Manduca sexta [Lepidoptera] »  ![Wikipedia: Manduca sexta [Lepidoptera]](/images/wikipedia.png)
|
|
| |
| Canonical SMILES | CC(CCCC(C)(C)O)C1CCC2(C1(CCC3C2=CC(=O)C4C3(CC(C(C4)O)O)C)C)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@H](C)CCCC(C)(C)O)O)C)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,13R,14S,17R)-2,3,14-trihydroxy-17-[(2R)-6-hydroxy-6-methylheptan-2-yl]-10,13-dimethyl-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | 34209-85-3 | | PubChem CID | 21124802 | InChiKey
[ ChemIDPlus: search ] | KMHXXYXMOIOEMU-BXVOCSMGSA-N | | InChI | InChI=1S/C27H44O5/c1-16(7-6-10-24(2,3)31)17-9-12-27(32)19-13-21(28)20-14-22(29)23(30)15-25(20,4)18(19)8-11-26(17,27)5/h13,16-18,20,22-23,29-32H,6-12,14-15H2,1-5H3/t16-,17-,18+,20+,22-,23+,25-,26-,27-/m1/s1 |
| |
| CI-MS (NH3) m/z | | | EI-MS m/z (relative intensity %) | 448 (3) (M)+, 430 (41), 420 (16), 412 (79), 402 (98), 397 (57), 384 (26), 379 (24), 369 (20), 357 (13), 341 (35), 331 (28), 327 (45), 301 (68), 300 (45), 283 (33), 277 (31), 276 (29), 249 (100), 231 (52), 223 (37), 213 (26), 201 (27), 199 (32), 185 (32), 173 (52), 161 (45), 147 (63), 145 (60), 131 (45), 121 (56), 109 (62), 91 (61), 81(77), 69 (94), 55 (98) with metastables at 385, 367, 362, 266 and 214.5 | | HR-MS | |
|
|
| C5D5N | | 01 | | | 02 | | | 03 | | | 04 | | | 05 | | | 06 | | | 07 | | | 08 | | | 09 | | | 10 | | | 11 | | | 12 | | | 13 | | | 14 | | | 15 | | | 16 | | | 17 | | | 18 | | | 19 | | | 20 | | | 21 | | | 22 | | | 23 | | | 24 | | | 25 | | | 26 | | | 27 | |
|
| C5D5N | | 01-Ha | | | 01-He | | | 02-Ha | | | 03-He | | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | | | 09-Ha | | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 0.73 | | 19-Me | 1.08 | | 21-Me | 0.99 and 1.08 | | 22-Ha | | | 22-Hb | | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.40 | | 27-Me | 1.40 |
| CDCl3 (2,3-OAc) | | 01-Ha | | | 01-He | | | 02-Ha | | | 03-He | | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | | | 09-Ha | | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 0.69 | | 19-Me | 1.04 | | 21-Me | 0.89 and 1.00 | | 22-Ha | | | 22-Hb | | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.23 | | 27-Me | 1.23 |
|
| |
| M.P. | 216-217 °C ; | | [α]D20 | | | IR (KBr) ν max (cm-1) | | | UV (EtOH) λ max (log ε) | 245 (4.070) ; |
| |
| HPTLC | | | TLC | | | GLC | | | HPLC-RP | column Spherisorb 5-ODS-2, solvent linear gradient (in 30 min) of 20% to 100% ACN-iPrOH (5:2) in 0.1% TFA in water, flow-rate 1 ml.min-1, Rt 21.8 min (2DE : 17.0 min) | | HPLC | NP-HPLC,(Zorbax-SIL) solvent CH2Cl2-iPrOH-H2O (125:40:3), flow-rate 1 ml.min-1, Rt 7.0 min |
| |
Drosophila melanogaster BII cell assay: EC50 = ca. 10-5M | Calliphora assay: 5% (20-hydroxyecdysone = 100%) |
| |
| |
Permanent link to this datasheet: 22-DEOXYECDYSONE
|
|




![Wikipedia: Manduca sexta [Lepidoptera]](/images/wikipedia.png)

