|
|
|
|
Year of first isolation: |
2024 |
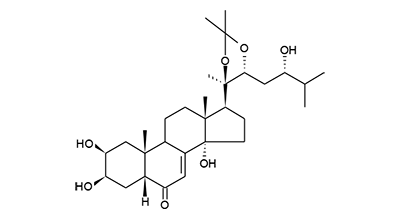
| Formula: | C30H48O7 |
| Molecular weight: | 520 |
| Occurence in plants: |
Acrostichum aureum L. (ferns) » 
|
| Occurence in animals: |
|
|
|
| |
| Canonical SMILES | | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C[C@@]12[C@@](C(C=C3C2CC[C@@]4(C)[C@@]3(O)CC[C@]4([H])[C@]([C@H]5C[C@H](O)C(C)C)(OC(C)(C)O5)C)=O)([H])C[C@@H](O)[C@@H](O)C1 » 
| | IUPAC Name | (2S,3R,5R,10R,13R,14S,17S)-2,3,14-trihydroxy-17-((4R,5R)-5-((S)-2-hydroxy-3-methylbutyl)-2,2,4-trimethyl-1,3-dioxolan-4-yl)-10,13-dimethyl-1,2,3,4,5,9,10,11,12,13,14,15,16,17-tetradecahydro-6H-cyclopenta[a]phenanthren-6-one | | CAS-RN | | | PubChem CID | | InChiKey
[ ChemIDPlus: search ] | DANMDDGJQVSALX-PQYIRGFSSA-N | | InChI | InChI=1S/C30H48O7/c1-16(2)20(31)14-25-29(7,37-26(3,4)36-25)24-9-11-30(35)18-12-21(32)19-13-22(33)23(34)15-27(19,5)17(18)8-10-28(24,30)6/h12,16-17,19-20,22-25,31,33-35H,8-11,13-15H2,1-7H3/t17?,19-,20-,22+,23-,24-,25+,27+,28+,29+,30+/m0/s1 |
| |
| HRESIMS (negative ion mode) m/z | 543.3272 [M+Na]+ , calculated 543.3292 for C30H48O7Na |
|
|
| ACETONE-D6 | | 01 | 37.4 | | 02 | 68.1 | | 03 | 68.0 | | 04 | 32.0 | | 05 | 51.1 | | 06 | 203.9 | | 07 | 121.8 | | 08 | 165.2 | | 09 | 34.4 | | 10 | 38.6 | | 11 | 21.0 | | 12 | 31.8 | | 13 | 48.0 | | 14 | 84.7 | | 15 | 31.4 | | 16 | 22.0 | | 17 | 49.9 | | 18 | 17.5 | | 19 | 24.3 | | 20 | 85.5 | | 21 | 22.4 | | 22 | 80.4 | | 23 | 33.8 | | 24 | 75.1 | | 25 | 33.7 | | 26 | 17.4 | | 27 | 19.3 | | 28 | 107.6 | | 29 | 27.1 | | 30 | 29.2 |
|
| ACETONE-D6 | | 01-Ha | 1.77 (m) | | 01-Hb | 1.39 (m) | | 02-Ha | 3.83 (brd, 10.5) | | 03-He | 3.92 (m) | | 04-Ha | 1.65 (m) | | 04-Hb | 1.65 (m) | | 05-H | 2.32 (dd, 6.0, 11.0) | | 07-H | 5.73 (d, 2.5) | | 09-H | 3.14 (ddd, 2.5, 7.0, 11.5) | | 11-Ha | 1?79 (m) | | 11-Hb | 1.65 (m) | | 12-Ha | 2.14 (td, 5.0, 13.0) | | 12-Hb | 1.79 (m) | | 15-Ha | 1.92 (m) | | 15-Hb | 1.67 (m) | | 16-Ha | 2.04 (m) | | 16-Hb | 1.98 (m) | | 17-H | 2.34 (overlapped) | | 18-Me | 0.81 (s) | | 19-Me | 0.91 (s) | | 21-Me | 1.17 (s) | | 22-H | 3.93 (dd, 4.0, 8.5) | | 23-Ha | 1.61 (m) | | 23-Hb | 1.61 (m) | | 24-H | 3.52 (dd, 3.0, 7.5) | | 25-H | 1.67 (m) | | 26-Me | 0.89 (d, 7.0) | | 27-Me | 0.90 (d, 7.0) | | 29-Me | 1.31 (s) | | 30-Me | 1.36 (s) |
|
| |
| M.P. | — °C ; | | [α]D25 | +70.6 (c 0.1 ; MeOH) | | IR (KBr) ν max (cm-1) | | | UV (MeOH) λ max (log ε) | (-) ; |
| |
| |
| |
| |
Permanent link to this datasheet: PTEROSTERONE 20,22-ACETONIDE
|
|





