|
|
|
|
Year of first isolation: |
1986 |
| Formula: | C34H48O8 |
| Molecular weight: | 584 |
| Occurence in plants: |
Silene scabrifolia [Caryophyllaceae] »  ![Wikipedia: Silene scabrifolia [Caryophyllaceae]](/images/wikipedia.png)
Silene otites [Caryophyllaceae] »  ![Wikipedia: Silene otites [Caryophyllaceae]](/images/wikipedia.png)
Silene tatarica [Caryophyllaceae] »  ![Wikipedia: Silene tatarica [Caryophyllaceae]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
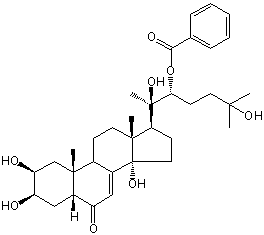
| |
| Canonical SMILES | CC12CCC3C(=CC(=O)C4C3(CC(C(C4)O)O)C)C1(CCC2C(C)(C(CCC(C)(C)O)OC(=O)C5=CC=CC=C5)O)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@](C)([C@@H](CCC(C)(C)O)OC(=O)c1ccccc1)O)O)C)C » 
| | IUPAC Name | [(2R,3R)-2,6-dihydroxy-6-methyl-2-[(2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-6-oxo-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-17-yl]heptan-3-yl] benzoate | | CAS-RN | | | PubChem CID | 11753556 | InChiKey
[ ChemIDPlus: search ] | GHZYCHXISZLQFT-WSZYVKLESA-N | | InChI | InChI=1S/C34H48O8/c1-30(2,39)14-13-28(42-29(38)20-9-7-6-8-10-20)33(5,40)27-12-16-34(41)22-17-24(35)23-18-25(36)26(37)19-31(23,3)21(22)11-15-32(27,34)4/h6-10,17,21,23,25-28,36-37,39-41H,11-16,18-19H2,1-5H3/t21-,23-,25+,26-,27-,28+,31+,32+,33+,34+/m0/s1 |
| |
| CI-MS (NH3) m/z | 585 (MH)+, 567, 549, 463, 445, 427, 363, 345 | | EI-MS m/z (relative intensity %) | 584 (M)+ (0.02), 566 (0.1), 548 (0.2), 533 (0.4), 530 (0.3), 462 (0.8), 445 (7), 444 (23), 429 (23), 427 (39), 426 (84), 411 (56), 408 (23), 393 (24), 375 (24), 363 (39), 357 (24), 345 (24), 329 (24), 327 (23), 315 (24), 301 (56), 300 (54), 251 (39), 250 (84), 249 (80), 232 (24), 231 (24), 152 (28), 126 (81), 122 (81), 109 (56), 105 (100), 99 (52), 81 (52), 77 (53), 69 (52), 59 (52), 51 (53), 43 (53). |
|
|
| C5D5N | | 01 | 37.9 | | 02 | 68.1 | | 03 | 68.1 | | 04 | 32.4 | | 05 | 51.3 | | 06 | 203.4 | | 07 | 121.8 | | 08 | 165.8 | | 09 | 34.4 | | 10 | 38.7 | | 11 | 21.6 | | 12 | 31.7 | | 13 | 48.2 | | 14 | 84.1 | | 15 | 32.0 | | 16 | 22.5 | | 17 | 50.6 | | 18 | 17.8 | | 19 | 24.4 | | 20 | 76.6 | | 21 | 21.1 | | 22 | 81.3 | | 23 | 26.2 | | 24 | 41.8 | | 25 | 69.2 | | 26 | 29.6 | | 27 | 30.0 | | contin. | 3', 5' 128.7; 4´ 133.0) | | O-C=O | 167.0 (1' 131.8; 2', 6' 130.0; |
|
| CD3OD | | 01-Ha | 1.45 | | 01-He | | | 02-Ha | 3.84 (ddd, 12, 3, 3) | | 03-He | 3.94 (ddd, 3, 3, 3) | | 04-Ha | | | 04-He | | | 05-H | 2.38 (dd, 5, 12) | | 07-H | 5.81 (d, 2) | | 09-Ha | 3.14 (m, w1/2=22) | | 11-Ha | | | 11-He | | | 12-Ha | 2.17 (ddd, 13, 13, 5) | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | 2.48 (t, 8.5) | | 18-Me | 0.89 (s ) | | 19-Me | 0.96 (s ) | | 21-Me | 1.42 (s ) | | 22-H | 5.15 (dd, 12, 2) | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.15 (s ) | | 27-Me | 1.16 (s ) | | C6H5-CO- m | 7.49 (t, 7) (2H) | | C6H5-CO- o | 8.08 (t, 7) (2H) | | C6H5-CO- p | 7.61 (t, 7) (1H) |
| C5D5N | | 01-Ha | | | 01-He | | | 02-Ha | 4.0-4.3 | | 03-He | 4.0-4.3 | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | 6.22 | | 09-Ha | 3.60 | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 1.21 | | 19-Me | 1.10 | | 21-Me | 1.78 | | 22-H | 5.76 | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.32 | | 27-Me | 1.32 | | C6H5-CO- m | 7.40 | | C6H5-CO- o | 8.27 | | C6H5-CO- p | 7,40 |
|
| |
| M.P. | 202-205 °C | | [α]D20 | + 45.0 ? 2° (c 1.1; MeOH) | | IR (KBr) ν max (cm-1) | 3420-3470 (OH), 1660 (C=O), 1710, 1285 (ester), 1610, 1587, | | UV (EtOH) λ max (log ε) | 235 (4.36) |
| |
| HPTLC | | | TLC | Rf 0.22 (EtOAc-MeOH-NH4OH, 85:10:5) (20E 0.18); Rf 0.28 (CH2Cl2-EtOH, 85:15) (20E 0.15) | | GLC | | | HPLC | 1- Retention time 10.9 min [ Zorbax-Sil 250 mm x 4.6 mm i.d., solvent CH2Cl2-iPrOH-H2O 125:40:3 v/v/v, flow-rate 1 ml.min-1] (20E : 16.8 min) 2- Retention time 22.8 min [ Zorbax-Sil 250 mm x 4.6 mm i.d., solvent CH2Cl2-iPrOH-H2O 125:25:2 v/v/v, flow-rate 1 ml.min-1] (20E 37.4 min) |
| |
Drosophila melanogaster BII cell assay: EC50 = 4.3 x 10-9M | Galleria mellonella in vivo assay: ED50 = 7.8 ug/g | Sarcophaga bullata in vivo assay: ED50 = 2.6 ug/g |
| |
| First isolation | SAATOV, Z. et al. (1986) Khim. Prir. Soedin., 77-80 |
 | | General | SAATOV, Z. et al. (1990) Khim. Prir. Soedin., 363-366 |
 | | General | GIRAULT, J.-P. et al. (1990) J. Nat. Prod. 53, 279-293 |
 | | Bioactivities | SLÁMA, K. et al. (1993) Insect Biochem. Molec. Biol. 23, 181-185 |
 | | Bioactivities | RAVI, M. et al. (2001) J. Chem. Inf. Comput. Sci. 41, 1587-1604 |
 |
| |
Permanent link to this datasheet: 20-HYDROXYECDYSONE 22-BENZOATE
|
|




![Wikipedia: Silene scabrifolia [Caryophyllaceae]](/images/wikipedia.png)

