|
|
|
|
Year of first isolation: |
1954 |
| Formula: | C27H44O6 |
| Molecular weight: | 464 |
| Occurence in plants: |
Pharbitis purpurea [Convolvulaceae] »  ![Wikipedia: Pharbitis purpurea [Convolvulaceae]](/images/wikipedia.png)
Pteridium aquilinum [Dennstaedtiaceae] »  ![Wikipedia: Pteridium aquilinum [Dennstaedtiaceae]](/images/wikipedia.png)
Polypodium vulgare [Polypodiaceae] »  ![Wikipedia: Polypodium vulgare [Polypodiaceae]](/images/wikipedia.png)
Osmunda asiatica [Osmundaceae] »  ![Wikipedia: Osmunda asiatica [Osmundaceae]](/images/wikipedia.png)
Osmunda japonica [Osmundaceae] »  ![Wikipedia: Osmunda japonica [Osmundaceae]](/images/wikipedia.png)
Blechnum minus [Blechnaceae] »  ![Wikipedia: Blechnum minus [Blechnaceae]](/images/wikipedia.png)
Angiosperms [Plantae] »  ![Wikipedia: Angiosperms [Plantae]](/images/wikipedia.png)
Ipomoea calonyction [Convolvulaceae] »  ![Wikipedia: Ipomoea calonyction [Convolvulaceae]](/images/wikipedia.png)
|
| Occurence in animals: |
Bombyx mori [Bombycidae] »  ![Wikipedia: Bombyx mori [Bombycidae]](/images/wikipedia.png)
Manduca sexta [Lepidoptera] »  ![Wikipedia: Manduca sexta [Lepidoptera]](/images/wikipedia.png)
various Insects [Insecta] »  ![Wikipedia: various Insects [Insecta]](/images/wikipedia.png)
|
|
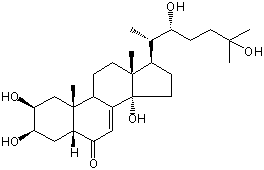
| |
| Canonical SMILES | CC(C1CCC2(C1(CCC3C2=CC(=O)C4C3(CC(C(C4)O)O)C)C)O)C(CCC(C)(C)O)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@H](C)[C@@H](CCC(C)(C)O)O)O)C)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,13R,14S,17R)-17-[(2S,3R)-3,6-dihydroxy-6-methylheptan-2-yl]-2,3,14-trihydroxy-10,13-dimethyl-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | 3604-87-3 | | PubChem CID | 19212 | InChiKey
[ ChemIDPlus: search ] | UPEZCKBFRMILAV-JMZLNJERSA-N | | InChI | InChI=1S/C27H44O6/c1-15(20(28)8-9-24(2,3)32)16-7-11-27(33)18-12-21(29)19-13-22(30)23(31)14-25(19,4)17(18)6-10-26(16,27)5/h12,15-17,19-20,22-23,28,30-33H,6-11,13-14H2,1-5H3/t15-,16+,17-,19-,20+,22+,23-,25+,26+,27+/m0/s1 |
| |
| CI-MS (NH3) m/z | 482 (M+H+NH3)+, 465 (MH)+, 447, 429, 427, 413, 411, 409, 395, 349, 323, 321, 315, 313 | | EI-MS m/z (relative intensity %) | 446 (3), 428 (29), 413 (2), 410 (3), 359 (4), 348 (8), 330 (15), 300 (23), 99 (100), 81 (61). | | FAB-MS m/z | 463 (M-H)- |
|
|
| C5D5N | | 01 | 38.08 (t ) | | 02 | 68.10 (d ) | | 03 | 68.10 (d ) | | 04 | 32.45 (t ) | | 05 | 51.41 (d ) | | 06 | 203.36 (s ) | | 07 | 121.61 (d ) | | 08 | 165.53 (s ) | | 09 | 34.63 (d ) | | 10 | 38.76 (s ) | | 11 | 21.20 (t ) | | 12 | 31.49 (t ) | | 13 | 47.70 (s ) | | 14 | 83.97 (s ) | | 15 | 32.03 (t ) | | 16 | 26.74 (t ) | | 17 | 48.28 (d ) | | 18 | 15.89 (q ) | | 19 | 24.55 (q ) | | 20 | 43.04 (d ) | | 21 | 13.74 (q ) | | 22 | 74.07 (d ) | | 23 | 25.69 (t ) | | 24 | 42.55 (t ) | | 25 | 69.80 (s ) | | 26 | 30.09 (q ) | | 27 | 30.01 (q ) |
| CD3OD | | 01 | 37.33 | | 02 | 68.69 | | 03 | 68.50 | | 04 | 32.89 | | 05 | 51.79 | | 06 | 206.55 | | 07 | 122.01 | | 08 | 167.63 | | 09 | 35.23 | | 10 | 39.24 | | 11 | 21.57 | | 12 | 32.03 | | 13 | 48.11 | | 14 | 85.07 | | 15 | 32.07 | | 16 | 27.00 | | 17 | 48.79 | | 18 | 16.18 | | 19 | 24.46 | | 20 | 43.46 | | 21 | 13.22 | | 22 | 75.24 | | 23 | 25.30 | | 24 | 42.25 | | 25 | 71.41 | | 26 | 29.05 | | 27 | 29.61 |
|
| C5D5N | | 01-Ha | 1.9 | | 01-He | 2.1 | | 02-Ha | 4.15 (m, w1/2=22) | | 03-He | 4.21 (m, w1/2=8) | | 04-Ha | 1.80 | | 04-He | 2.03 | | 05-H | 3.01 (dd, 13, 4) | | 07-H | 6.25 (d, 2.5) | | 09-Ha | 3.54 (m, w1/2=22) | | 11-Ha | 1.60 | | 11-He | 1.8 | | 12-Ha | 2.53 | | 12-He | 1.83 | | 15-Ha | 1.85* | | 15-Hb | 1.75* | | 16-Ha | 2.19$ | | 16-Hb | 1.59$ | | 17-H | 2.51 | | 18-Me | 0.70 (s ) | | 19-Me | 1.06 (s ) | | 20-H | 2.12 | | 21-Me | 1.28 (d, 7) | | 22-H | 4.05 | | 23-Ha | 1.80 | | 23-Hb | 1.94 | | 24-Ha | 2.26 | | 24-Hb | 1.79 | | 26-Me | 1.37 (s ) | | 27-Me | 1.37 (s ) |
| CD3OD | | 01-Ha | 1.43 | | 01-He | 1.78 | | 02-Ha | 3.83 (ddd, 12, 3, 3) | | 03-He | 3.94 (ddd, 3, 3, 3) | | 04-Ha | 1.65 | | 04-He | 1.75 | | 05-H | 2.38 (dd, 12, 5) | | 07-H | 5.81 (d, 2.5) | | 09-Ha | 3.14 (m, w1/2=22) | | 11-Ha | 1.65 | | 11-He | 1.78 | | 12-Ha | 2.10 (ddd, 13,13,5) | | 12-He | 1.7-1.8 | | 15-Ha | 2.00 | | 15-Hb | 1.53 | | 16-Ha | 1.98° | | 16-Hb | 1.48° | | 17-H | 2.01 | | 18-Me | 0.73 (s ) | | 19-Me | 0.97 (s ) | | 20-H | 1.72 | | 21-Me | 0.95 (d, 7.5) | | 22-H | 3.59 (m, w1/2=16) | | 23-Ha | 1.30 | | 23-Hb | 1.60 | | 24-Ha | 1.75 | | 24-Hb | 1.45 | | 26-Me | 1.19 (s ) | | 27-Me | 1.20 (s ) |
|
| |
| M.P. | 237-239 °C | | [α]D20 | + 64.7 ° (c ; EtOH) | | IR (KBr) ν max (cm-1) | 3333 (OH), 1657 (cyclohexenone) | | UV (EtOH) λ max (log ε) | 242 (4.093) |
| |
| HPTLC | | | TLC | NP-TLC on silicagel Rf 0.21 (CHCl3-EtOH 4:1); RP-TLC on paraffin coated silica gel Rf 0.37 (MeOH-H2O 50:50); RP-TLC on C18-bonded silica gel Rf 0.37 (MeOH-H2O 65:35) | | GLC | Ret 5.85 min on 1.5% OV101 (0.9 m x 4.5 mm i.d.) at 285°C after silylation at 140°C for 60 h, presumed 2,3,14,22,25 pentakis-TMS ether. (many systems are available - only examples are given here) | | HPLC | |
| |
Drosophila melanogaster BII cell assay: EC50 = 1.1 x 10-6M | Drosophila melanogaster BII cell assay: EC50 = 1.0 x 10-6M | Drosophila melanogaster imaginal disc assay: EC50 = 1.5 x 10-5M | Drosophila melanogaster imaginal disc assay: EC50 = 2.2 x 10-5M | Drosophila melanogaster Kc-H cell assay: EC50 = 2.3 x 10-6M | Dermestes vulpinus in vivo assay: ED50 = 42.0 ug/g | Galleria mellonella in vivo assay: ED50 = 7.8 ug/g | Sarcophaga bullata in vivo assay: ED50 = 4.2 ug/g | Calliphora bioassay: 100% (20-hydroxyecdysone = 100%) |
| |
| First isolation | BUTEN, T et al. (1954) Z. Naturforsch. 9B, 389-391 |
 | | General | KARLSON, P. et al. (1965) Chem. Ber. 98, 2394-2402 |
 | | General | HUBER, R. et al. (1965) Chem. Ber. 98, 2403-2424 |
 | | General | FURLENMEIER, A. et al. (1966) Helv. Chim. Acta 49, 1591-1606 |
 | | General | SIDDALL, J.B. et al. (1966) J. Am. Chem. Soc. 88, 862-863 |
 | | First isolation | HEINRICH, G. et al. (1967) Experientia 23, 995 |
 | | Bioactivities | CHIHARA, C.J. et al. (1972) J. Insect Physiol. 18, 1115-1123 |
 | | Bioactivities | CHERBAS, L. et al. (1980) W. Roux Arch. Devel. Biol. 189, 1-15 |
 | | Bioactivities | BERGAMASCO, R. et al. (1980) In: Progress in Ecdysone Research (Ed. J.A. Hoffmann), Elsevier/North Holland Biomed. Press, Amsterdam 299-324 |
 | | Bioactivities | YUND, M.A. et al. (1980) In: Invert. Systems in Vitro pp. 229-237 |
 | | General | WILSON, I.D. et al. (1985) J. Chromatogr. 318, 373-377 |
 | | General | BIELBY, C.R. et al. (1986) J. Chromatogr. 351, 57-64 |
 | | General | GIRAULT, J.-P. et al. (1988) J. Insect Physiol. 34, 701-706 |
 | | Bioactivities | SLÁMA, K. et al. (1993) Insect Biochem. Molec. Biol. 23, 181-185 |
 | | Bioactivities | DINAN, L. et al. (1999) J. Computer-aided Mol. Design 13, 185-207 |
 | | Bioactivities | DINAN, L. et al. (2003) In: Studies in Natural Products Chemisty (ed. Atta-ur-Rahman), Elsevier, Amsterdam, 29, 3-71 |
 | | Identification | BUDĚŠÍNSKÝ, M. et al. (2008) Steroids 73, 502-514 |
 |
| |
Permanent link to this datasheet: ECDYSONE
|
|




![Wikipedia: Pharbitis purpurea [Convolvulaceae]](/images/wikipedia.png)

