|
|
|
|
Year of first isolation: |
2010 |
| Formula: | C34H48O7 |
| Molecular weight: | 568 |
| Occurence in plants: |
Taxus canadensis [Taxaceae] »  ![Wikipedia: Taxus canadensis [Taxaceae]](/images/wikipedia.png)
|
| Occurence in animals: |
|
|
|
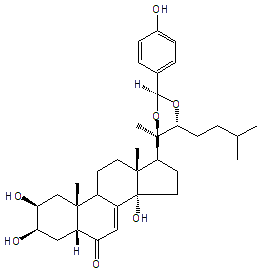
| |
| Canonical SMILES | CC(C)CCC1C(OC(O1)C2=CC=C(C=C2)O)(C)C3CCC4(C3(CCC5C4=CC(=O)C6C5(CC(C(C6)O)O)C)C)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@]1(C)[C@@H](CCC(C)C)O[C@@H](O1)c1ccc(cc1)O)O)C)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-17-[(4R,5R)-2-(4-hydroxyphenyl)-4-methyl-5-(3-methylbutyl)-1,3-dioxolan-4-yl]-10,13-dimethyl-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | | | PubChem CID | 102259738 | InChiKey
[ ChemIDPlus: search ] | UNGIQOPVKKKLMI-COJGYATGSA-N | | InChI | InChI=1S/C34H48O7/c1-19(2)6-11-29-33(5,41-30(40-29)20-7-9-21(35)10-8-20)28-13-15-34(39)23-16-25(36)24-17-26(37)27(38)18-31(24,3)22(23)12-14-32(28,34)4/h7-10,16,19,22,24,26-30,35,37-39H,6,11-15,17-18H2,1-5H3/t22-,24-,26+,27-,28-,29+,30?,31+,32+,33+,34+/m0/s1 |
| |
| EI-MS m/z (relative intensity %) | | | HR-MS ((+)-FAB) m/z | 569.3473 (calculated 569.3473 for C34H49O7, [M+H] +), 551.3474 (calculated 551.3467 for C34H47O6, [M+H-H2O] +) | | CI-MS (NH3) m/z | |
|
|
| CD3OD | | 01 | 37.37 | | 02 | 68.72 | | 03 | 68.51 | | 04 | 32.88 | | 05 | 51.79 | | 06 | 206.41 | | 07 | 122.18 | | 08 | 167.54 | | 09 | 35.17 | | 10 | 39.24 | | 11 | 21.51 | | 12 | 32.19 | | 13 | 48.23 | | 14 | 85.28 | | 15 | 31.78 | | 16 | 22.80 | | 17 | 51.55 | | 18 | 17.67 | | 19 | 24.45 | | 20 | 85.52 | | 21 | 23.58 | | 22 | 85.75 | | 23 | 27.72 | | 24 | 37.60 | | 25 | 29.25 | | 26 | 22.95 | | 27 | 22.87 | | 28 | 105.23 | | 29 | 131.03 | | 30 (30') | 129.47 | | 31 (31') | 115.83 | | 32 | 159.38 |
|
| CD3OD | | 01-Ha | 1.45 (t, 13.0) | | 01-He | 1.81 (m) | | 02-Ha | 3.85 (m) | | 03-He | 3.97 (br, s) | | 04-Ha | 1.73 (m) | | 04-He | 1.76 (m) | | 05-H | 2.38 (dd, 12.6, 4.3) | | 07-H | 5.84, (d, 2.0) | | 09-H | 3.18 (m) | | 11-Ha | 1.72 (m) | | 11-He | 1.83 (m) | | 12-Ha | 2.16 (td, 13.4, 4.8) | | 12-He | 1.87 (br, dd) | | 15-Ha | 1.68 (m) | | 15-Hb | 2.02 (m) | | 16-Ha | 2.09 (m) | | 16-Hb | 1.99 (m) | | 17-H | 2.44 (t, 9.0) | | 18-Me | 0.90 (s) | | 19-Me | 0.98 (s) | | 21-Me | 1.29 (s) | | 22-H | 3.85 (d) | | 23-Ha | 1.54 (m) | | 23-Hb | 1.61 (m) | | 24-Ha | 1.49 (m) | | 24-Hb | 1.32 (m) | | 25-H | 1.63 (m) | | 26-Me | 0.95 (d, 6.6) | | 27-Me | 0.95 (d, 6.6) |
|
| |
| M.P. | °C ; | | [α]D22 | + 64 ° (c = 0.10, MeOH) | | IR (KBr) ν max (cm-1) | | | UV (EtOH) λ max (log ε) | () ; |
| |
| HPLC | Whatman Partisil 10 ODS-2Mag-20 preparative column (500 mm long, 22 mm i.d.) eluted with a linear gradient of acetonitrile in water, 25% to 100% in 50 min, flow-rate 18 mL/min. | | GLC | | | HPTLC | | | TLC | |
| |
| |
| |
Permanent link to this datasheet: PONASTERONE A 20,22-p-HYDROXY-BENZYLIDENE ACETAL
|
|




![Wikipedia: Taxus canadensis [Taxaceae]](/images/wikipedia.png)

