|
|
|
|
Year of first isolation: |
1982 |
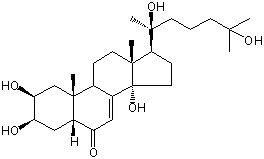
| Formula: | C27H44O6 |
| Molecular weight: | 464 |
| Occurence in plants: |
Taxus cuspidata [Taxaceae] »  ![Wikipedia: Taxus cuspidata [Taxaceae]](/images/wikipedia.png)
Silene nutans [Caryophyllaceae] »  ![Wikipedia: Silene nutans [Caryophyllaceae]](/images/wikipedia.png)
Lychnis flos-culculi [Caryophyllaceae] »  ![Wikipedia: Lychnis flos-culculi [Caryophyllaceae]](/images/wikipedia.png)
Leuzea carthamoides [Asteraceae] »  ![Wikipedia: Leuzea carthamoides [Asteraceae]](/images/wikipedia.png)
|
| Occurence in animals: |
Pycnogonum litorale [pantopod] »  ![Wikipedia: Pycnogonum litorale [pantopod]](/images/wikipedia.png)
Bombyx mori [Bombycidae] »  ![Wikipedia: Bombyx mori [Bombycidae]](/images/wikipedia.png)
|
|
| |
| Canonical SMILES | CC12CCC3C(=CC(=O)C4C3(CC(C(C4)O)O)C)C1(CCC2C(C)(CCCC(C)(C)O)O)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@](C)(CCCC(C)(C)O)O)O)C)C » 
| | IUPAC Name | (2S,3R,5R,9R,10R,13R,14S,17S)-17-[(2S)-2,6-dihydroxy-6-methylheptan-2-yl]-2,3,14-trihydroxy-10,13-dimethyl-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-6-one | | CAS-RN | 19536-24-4 | | PubChem CID | 15251158 | InChiKey
[ ChemIDPlus: search ] | KFLDRYHMXLUSFO-ISSBNPATSA-N | | InChI | InChI=1S/C27H44O6/c1-23(2,31)9-6-10-26(5,32)22-8-12-27(33)17-13-19(28)18-14-20(29)21(30)15-24(18,3)16(17)7-11-25(22,27)4/h13,16,18,20-22,29-33H,6-12,14-15H2,1-5H3/t16-,18-,20+,21-,22-,24+,25+,26-,27+/m0/s1 |
| |
| CI-MS (NH3) m/z | 482 (M+H+NH3)+, 465 (M+H)+, 447, 429, 411 | | EI-MS m/z (relative intensity %) | 464 (M)+ (0.3), 446 (1.4), 431 (0.8), 428 (35), 418 (1), 413 (16), 410 (28), 400 (2.5), 395 (12), 385 (0.7), 343 (5), 327 (54), 325 (20), 320 (20), 309 (10), 302, 301 (19), 300 (35), 299 (27), 250 (30), 249 (15), 145 (20), 127 (97), 109 (100), 81 (20), 69 (54) | | FAB-MS | 487 (2) [M+Na]+, 465 (6) [M+H]+, 447 (11), 429 (12), 411 (10), 397 (3), 371 (3), 355 (5), 303 (10), 301 (12), 249 (10), 213 (11), 181 (16), 165 (19), 145 (21), 128 (27), 109 (48), 91 (65), 69 (100) |
|
|
| CD3OD | | 01 | 37.37 | | 02 | 68.72 | | 03 | 68.53 | | 04 | 32.86 | | 05 | 51.80 | | 06 | 206.51 | | 07 | 122.10 | | 08 | 168.14 | | 09 | 35.05 | | 10 | 39.26 | | 11 | 21.51 | | 12 | 32.38 | | 13 | 48.07 | | 14 | 85.54 | | 15 | 31.58 | | 16 | 21.96 | | 17 | 53.36 | | 18 | 18.12 | | 19 | 24.39 | | 20 | 75.99 | | 21 | 26.46 | | 22 | 45.89 | | 23 | 20.10 | | 24 | 45.50 | | 25 | 71.48 | | 26 | 29.33 | | 27 | 29.10 |
| CDCl3 | | 01 | 38.4 (t ) | | 02 | 67.0 (d ) | | 03 | 68.7 (d ) | | 04 | 34.0 (t ) | | 05 | 51.0 (d ) | | 06 | 202.0 (s ) | | 07 | 121.5 (d ) | | 08 | 164.8 (s ) | | 09 | 33.6 (d ) | | 10 | 38.4 (s ) | | 11 | 20.4 (t ) | | 12 | 31.5 (t ) | | 13 | 46.9 (s ) | | 14 | 84.8 (s ) | | 15 | 30.9 (t ) | | 16 | 29.7 (t ) | | 17 | 52.2 (d ) | | 18 | 17.5 (q ) | | 19 | 23.8 (q ) | | 20 | 75.0 (s ) | | 21 | 26.5 (q ) | | 22 | 44.3 (t ) | | 23 | 18.7 (t ) | | 24 | 44.7 (t ) | | 25 | 71.0 (s ) | | 26 | 29.3 (q ) | | 27 | 29.5 (q ) |
|
| D2O | | 01-Ha | | | 01-Hb | | | 02-Ha | 3.90 (m, w1/2 = 21) | | 03-He | 4.07 (m, w1/2 = 8) | | 04-Ha | | | 04-He | | | 05-H | 2.34 (t like ) | | 07-H | 5.97 (d, 2.5) | | 09-Ha | 3.10 (m, w1/2 = 22) | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | 2.31 (m ) | | 18-Me | 0.82 (s ) | | 19-Me | 0.99 (s ) | | 21-Me | 1.30 (s ) | | 22-H | | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.22 (s ) | | 27-Me | 1.22 (s ) |
| CD3OD | | 01-Ha | 1.42 (dd 13.3, 12.0) | | 01-Hb | 1.79 (dd 13.3, 4.4) | | 02-Ha | 3.84 (ddd 3.2, ~3.0, ~3.0) | | 03-He | 3.95 (q) | | 04-Ha | 1.75 | | 04-He | 1.70 | | 05-H | 2.38 (dd 12.0, 5.5) | | 07-H | 5.81 | | 09-Ha | 3.15 (ddd 11.8, 7.0, 2.7) | | 11-Ha | 1.68 | | 11-He | 1.78 | | 12-Ha | 2.12 (dt) | | 12-He | 1.84 | | 15-Ha | 1.95 | | 15-Hb | 1.61 | | 16-Ha | 1.71 | | 16-Hb | 1.92 | | 17-H | 2.34 (dd) | | 18-Me | 0.86 (s) | | 19-Me | 0.96 (s) | | 21-Me | 1.28 (s) | | 22-H | 1.38 - 1.52 | | 23-Ha | 1.38 - 1.52 | | 23-He | 1.38 - 1.52 | | 24-Ha | 1.38 - 1.52 | | 24-Hb | 1.38 - 1.52 | | 26-Me | 1.19 (s) | | 27-Me | 1.19 (s) |
|
| |
| M.P. | 241-242 °C | | [α]D28 | + 80.9 ? 2 ° (c 0.42; MeOH) | | IR (KBr) ν max (cm-1) | 3400-3430 (OH), 1710 (CO), 1647 (cyclohexenone) | | UV (EtOH) λ max (log ε) | 244 (4.03) |
| |
| TLC | Rf 0.35 (CHCl3-MeOH-H2O 80:20:0.2) (20E 0.30); Rf 0.20 (EtOAc-MeOH-NH4OH 85:10:5) (20E : 0.18); Rf 0.17 (CH2Cl2-EtOH 85:15) (20E : 0.15) | | GLC | | | NP-HPLC | NP-HPLC, column Zorbax-Sil 250 mm x 4.6 mm i.d., , flow-rate 1 ml.min-1, solvent CH2Cl2-iPrOH-H2O (125:40:3) Ret 13.4 min (20E : 16.8 min); solvent CH2Cl2-iPrOH-H2O (125:25:2) Ret 28.3 min (20E 37.4 min). | | RP-HPLC | RP-HPLC, column Spherisorb-5ODS2 250 mm x 4.6 mm i.d., flow-rate 1 ml.min-1, solvent ACN/ 0.1% TFA in H2O (23:77), Ret 13.9 min (20E : 5.5 min) |
| |
Drosophila melanogaster BII cell assay: EC50 = 1.4 x 10-8M | Drosophila melanogaster BII cell assay: EC50 = 9.5 x 10-8M | Calliphora assay: 1-2% (20-hydroxyecdysone = 100%) |
| |
| General | GALBRAITH, M.N. et al. (1969) Aust. J. Chem. 22, 1517-1524 |
 | | General | KAPLANIS, J.N. et al. (1972) Steroids 20, 105-120 |
 | | Bioactivities | BERGAMASCO, R. et al. (1980) In: Progress in Ecdysone Research (Ed. J.A. Hoffmann), Elsevier/North Holland Biomed. Press, Amsterdam 299-324 |
 | | First isolation | NAKANO, K. et al. (1982) Phytochemistry 21, 2749-2751 |
 | | General | BALTAYEV, U.A. et al. (1985) Khim. Prir. Soedin. 62-66 |
 | | General | BÜCKMANN, D. et al. (1986) J. Comp. Physiol., B 47, 257-267 |
 | | General | GIRAULT, J.-P. et al. (1990) J. Nat. Prod. 53, 279-293 |
 | | General | KAMBA, M. et al. (1994) Insect Biochem. Molec. Biol. 24, 395-402 |
 | | Bioactivities | DINAN, L. et al. (1999) J. Computer-aided Mol. Design 13, 185-207 |
 | | Identification | VOKÁČ, K. et al. (2002) Collect. Czech. Chem. Commun. 67, 124-139 |
 |
| |
Permanent link to this datasheet: TAXISTERONE
|
|




![Wikipedia: Taxus cuspidata [Taxaceae]](/images/wikipedia.png)

