|
|
|
|
Year of first isolation: |
1986 |
| Formula: | C29H46O8 |
| Molecular weight: | 522 |
| Occurence in plants: |
Silene otites [Caryophyllaceae] »  ![Wikipedia: Silene otites [Caryophyllaceae]](/images/wikipedia.png)
Serratula coronata [Asteraceae] »  ![Wikipedia: Serratula coronata [Asteraceae]](/images/wikipedia.png)
|
| Occurence in animals: |
Cepaea nemoralis [Helicidae] »  ![Wikipedia: Cepaea nemoralis [Helicidae]](/images/wikipedia.png)
Pycnogonum litorale [pantopod] »  ![Wikipedia: Pycnogonum litorale [pantopod]](/images/wikipedia.png)
Drosophila melanogaster [Diptera] »  ![Wikipedia: Drosophila melanogaster [Diptera]](/images/wikipedia.png)
Chrysolina carnifex [Chrysomelidae] »  ![Wikipedia: Chrysolina carnifex [Chrysomelidae]](/images/wikipedia.png)
|
|
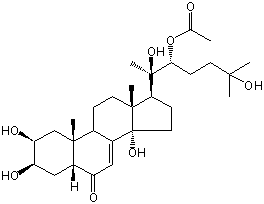
| |
| Canonical SMILES | CC(=O)OC(CCC(C)(C)O)C(C)(C1CCC2(C1(CCC3C2=CC(=O)C4C3(CC(C(C4)O)O)C)C)O)O | Isomeric SMILES
[ PubChem: search | XML ]
[ ChemSpider: search ] | C1[C@]2([C@@H](C[C@H]([C@H]1O)O)C(=O)C=C1[C@@H]2CC[C@]2([C@]1(CC[C@@H]2[C@](C)([C@@H](CCC(C)(C)O)OC(=O)C)O)O)C)C » 
| | IUPAC Name | [(2R,3R)-2,6-dihydroxy-6-methyl-2-[(2S,3R,5R,9R,10R,13R,14S,17S)-2,3,14-trihydroxy-10,13-dimethyl-6-oxo-2,3,4,5,9,11,12,15,16,17-decahydro-1H-cyclopenta[a]phenanthren-17-yl]heptan-3-yl] acetate | | CAS-RN | 22799-02-6 | | PubChem CID | 15607741 | InChiKey
[ ChemIDPlus: search ] | YAIZECBIJHIPCU-FORVDKSSSA-N | | InChI | InChI=1S/C29H46O8/c1-16(30)37-24(9-10-25(2,3)34)28(6,35)23-8-12-29(36)18-13-20(31)19-14-21(32)22(33)15-26(19,4)17(18)7-11-27(23,29)5/h13,17,19,21-24,32-36H,7-12,14-15H2,1-6H3/t17-,19-,21+,22-,23-,24+,26+,27+,28+,29+/m0/s1 |
| |
| CI-MS (NH3) m/z | 540 (M+H+NH3)+, 523, (MH)+, 505, 487, 463 (MH-60 (acetic acid))+, 445, 427, 409, 363 (MH-side-chain)+, 345 ... | | EI-MS m/z (relative intensity %) | | | HR-MS | |
|
|
| CD3OD | | 01 | 37.32 (t) | | 02 | 68.69 (d) | | 03 | 68.49 (d) | | 04 | 32.83 (t) | | 05 | 51.74 (d) | | 06 | 206.47 (s) | | 07 | 122.20 (d) | | 08 | 167.97 (s) | | 09 | 35.05 (d) | | 10 | 39.26 (s) | | 11 | 21.52 (t) | | 12 | 32.56 (t) | | 13 | ? | | 14 | 85.19 (s) | | 15 | 31.78 (t) | | 16 | 21.52 (d) | | 17 | 50.87 (d) | | 18 | 18.13 (q) | | 19 | 24.45 (q) | | 20 | 77.53 (s) | | 21 | 21.28 (q) | | 22 | 80.66 (d) | | 23 | 26.20 (t) | | 24 | 41.55 (t) | | 25 | 71.46 (s) | | 26 | 28.97 (q) | | 27 | 29.49 (q) | | MeCO | 21.28 (q), 173.42 (s) |
|
| CDCl3 | | 01-Ha | 1.4 | | 01-Hb | 1.85 | | 02-Ha | 3.88 (m ) | | 03-He | 4.03 (m ) | | 04-Ha | 1.6 | | 04-He | 1.85 | | 05-H | 2.42 (dd ) | | 07-H | 5.84 (d, 2.5) | | 09-Ha | 2.98 | | 11-Ha | 1.6 | | 11-He | 1.75 | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | 2.35 (m ) | | 18-Me | 0.79 (s ) | | 19-Me | 0.97 (s ) | | 21-Me | 1.20 (s ) | | 22-H | 4.84 | | 23-Ha | 1.5 | | 23-Hb | 1.7 | | 24-Ha | | | 24-Hb | | | 26-Me | 1.22 (s ) | | 27-Me | 1.26 (s ) | | Me-CO- | 2.10 (s) (3H) |
| D2O | | 01-Ha | | | 01-Hb | | | 02-Ha | 3.99 (m, w1/2=21) | | 03-He | 4.07 (m, w1/2=8) | | 04-Ha | | | 04-He | | | 05-H | | | 07-H | 5.97 (d ) | | 09-Ha | 3.11 (m, w1/2=22) | | 11-Ha | | | 11-He | | | 12-Ha | | | 12-He | | | 15-Ha | | | 15-Hb | | | 16-Ha | | | 16-Hb | | | 17-H | | | 18-Me | 0.86 (s ) | | 19-Me | 1.00 (s ) | | 21-Me | 1.34 (s ) | | 22-H | 4.85 (d, w1/2=14) | | 23-Ha | | | 23-Hb | | | 24-Ha | | | 24-Hb | | | 26-Me | 1.21 (s ) | | 27-Me | 1.21 (s ) | | Me-CO- | 2.16 (s) (3H) |
|
| |
| M.P. | 147-148 °C | | [α]D20 | + 49.5 (c 0,96; MeOH) | | IR (KBr) ν max (cm-1) | | | UV (EtOH) λ max (log ε) | |
| |
| HPTLC | | | TLC | Rf 0.19 (EtOAc-MeOH-NH4OH 85:10:5); Rf 0.23 (CH2Cl2-EtOH 85:15). | | GLC | | | NP-HPLC | (Merck Lichrosorb Si 60) Ret 12.2 ml (solvent CH2Cl2-iPrOH-H2O 125: 30: 2) (20E 27.6 ml) | | RP-HPLC | (Spherisorb-ODS2) Ret. 12.4 min (solvent ACN-0.1% TFA in water 23:77 (v/v), isocratic) (20E 5.5 min) |
| |
Drosophila melanogaster BII cell assay: EC50 = 2.0 x 10-7M | Drosophila melanogaster BII cell assay: EC50 = 4.0 x 10-6M | Musca assay: 76% (20-hydroxyecdysone = 100%) |
| |
| First isolation | GALBRAITH, M.N. et al. (1969) Aust. J. Chem. 22, 1045-1057 |
 | | General | BÁTHORI, M. et al. (1986) Herba Hungarica 25, 105-117 |
 | | Identification | BÜCKMANN, D. et al. (1986) J. Comp. Physiol., B 156, 759-765 |
 | | Struct. analysis | GARCIA, M. et al. (1986) Int. J. Invertebr. Reprod. Develop. 9, 43-58 |
 | | General | MAROY, P. et al. (1988) J. Insect Physiol. 34, 633-637 |
 | | Bioactivities | RAVI, M. et al. (2001) J. Chem. Inf. Comput. Sci. 41, 1587-1604 |
 | | Bioactivities | SUKSAMRARN, A. et al. (2002) Insect Biochem. Molec. Biol. 32, 193-197 |
 | | First isolation | LAURENT, P. et al. (2003) Chemoecology 13, 109-111 |
 | | Bioactivities | DINAN, L. et al. (2005) Eur. J. Entomol. 92, 271-283 |
 | | Bioactivities | DINAN, L. et al. (2005) In: Comprehensive Molecular Insect Science (eds. L. I. Gilbert, C. Iatrou, S. Gill), Elsevier, Oxford, III, 197-242 |
 |
| |
Permanent link to this datasheet: 20-HYDROXYECDYSONE 22-ACETATE
|
|




![Wikipedia: Silene otites [Caryophyllaceae]](/images/wikipedia.png)

